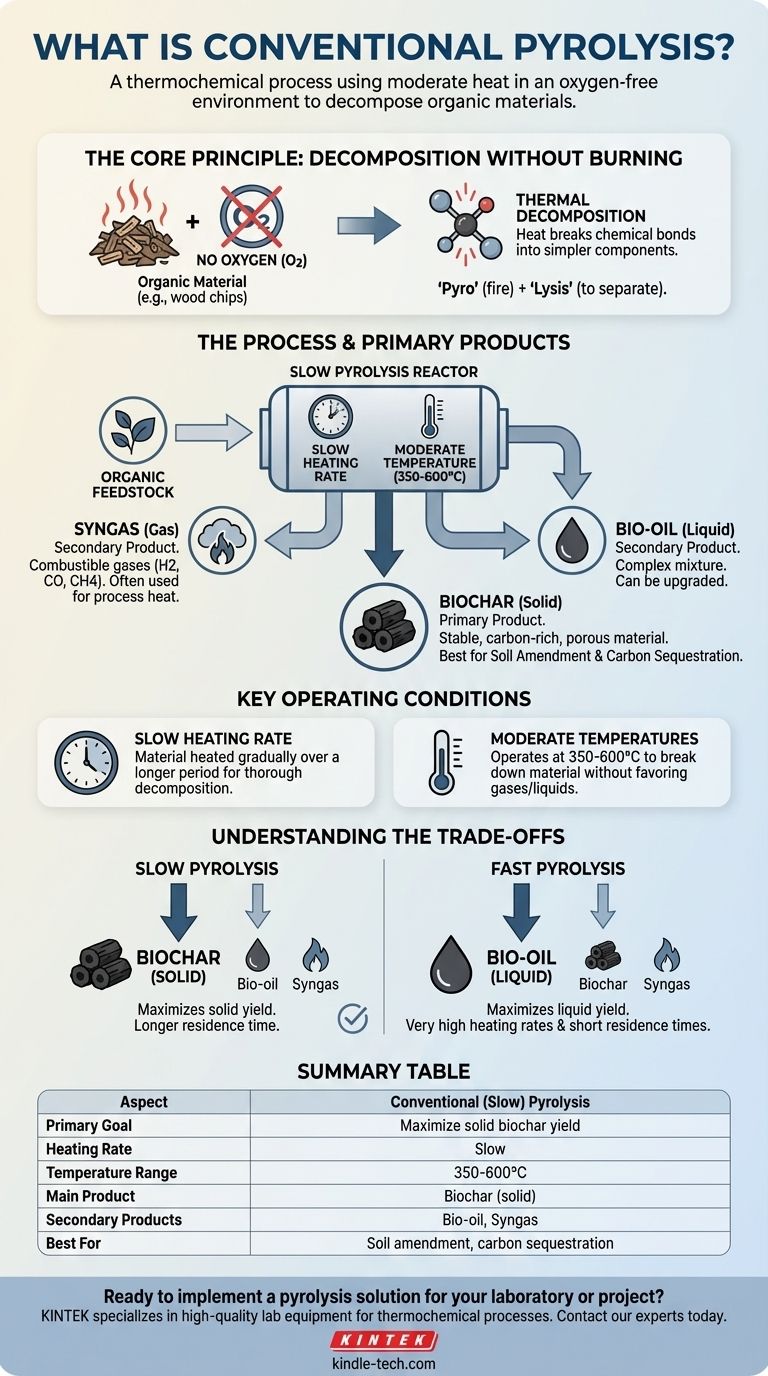
In essence, conventional pyrolysis is a thermochemical process that uses moderate heat in a completely oxygen-free environment to decompose organic materials. Rather than burning the material, this controlled heating breaks it down into a stable, carbon-rich solid (charcoal or biochar), a liquid (bio-oil), and a combustible gas (syngas). It is the oldest and most straightforward form of pyrolysis.
The central purpose of conventional pyrolysis is to slowly and deliberately transform organic matter, like wood or agricultural waste, with the primary goal of maximizing the yield of the solid charcoal product, known as biochar.

How Conventional Pyrolysis Works
Conventional pyrolysis, often called slow pyrolysis, is defined by its specific operating conditions. These conditions are deliberately chosen to favor the creation of a solid, stable end product over liquids or gases.
The Core Principle: Decomposition Without Burning
The key to pyrolysis is the absence of oxygen. Without oxygen, the material cannot combust or burn. Instead, the applied heat energy breaks the complex chemical bonds within the organic matter, deconstructing it into simpler, more stable components.
This process is a form of thermal decomposition, where heat alone drives the chemical separation. The Greek roots of the word—'pyro' (fire) and 'lysis' (to separate)—perfectly describe this action.
The Three Primary Products
The slow decomposition process reliably yields three distinct outputs, with proportions depending on the input material and process temperature.
- Biochar (Solid): This is the primary and most valuable product of conventional pyrolysis. It is a stable, porous, and carbon-dense material similar to charcoal.
- Bio-oil (Liquid): Also known as pyrolysis oil, this is a complex mixture of oxygenated organic compounds condensed from the vapor stream. In slow pyrolysis, this is a secondary product.
- Syngas (Gas): This is a mixture of non-condensable, combustible gases like hydrogen, carbon monoxide, and methane. It is often captured and used to provide the heat needed to sustain the pyrolysis reaction itself.
Key Operating Conditions
Conventional pyrolysis is distinguished from other methods by two main factors:
- Slow Heating Rate: The material is heated gradually over a longer period, allowing heat to penetrate fully and ensuring a thorough, even decomposition.
- Moderate Temperatures: The process typically operates in a temperature range of 350-600°C. This is hot enough to break down the material but not so hot that it aggressively favors gas or liquid production.
Understanding the Trade-offs
While effective, conventional pyrolysis involves a distinct set of compromises that make it suitable for some goals but not others.
Slow vs. Fast Pyrolysis
The main trade-off is between producing a solid or a liquid.
- Conventional (Slow) Pyrolysis: Maximizes the solid biochar yield. The slow heating gives carbon atoms time to arrange themselves into stable aromatic structures.
- Fast Pyrolysis: Uses very high heating rates and short residence times to maximize the liquid bio-oil yield by rapidly vaporizing the material before it can form char.
Process Speed and Throughput
As the name implies, slow pyrolysis is not a rapid process. The longer residence times required for the reactions mean that the throughput of a given reactor is lower compared to faster methods, which can impact industrial-scale economics.
Product Quality
The bio-oil produced during slow pyrolysis is often viscous and contains a high water content, making it difficult to use as a drop-in fuel without significant and costly upgrading. The primary focus remains on the quality and quantity of the biochar.
Making the Right Choice for Your Goal
Selecting the correct thermochemical process depends entirely on the end product you wish to create.
- If your primary focus is soil amendment or carbon sequestration: Conventional (slow) pyrolysis is the ideal method, as its main output is high-quality, stable biochar.
- If your primary focus is producing a liquid biofuel: You must look to fast pyrolysis, as it is specifically designed to maximize the bio-oil yield.
- If your primary focus is converting plastic waste to fuel: Both slow and fast pyrolysis can be used, but fast pyrolysis is generally preferred for maximizing the yield of liquid hydrocarbons.
By understanding these fundamental differences, you can effectively match the process to the desired outcome.
Summary Table:
| Aspect | Conventional (Slow) Pyrolysis |
|---|---|
| Primary Goal | Maximize solid biochar yield |
| Heating Rate | Slow |
| Temperature Range | 350-600°C |
| Main Product | Biochar (solid) |
| Secondary Products | Bio-oil, Syngas |
| Best For | Soil amendment, carbon sequestration |
Ready to implement a pyrolysis solution for your laboratory or project? KINTEK specializes in high-quality lab equipment and consumables for thermochemical processes. Whether you're researching biochar production or scaling up your operations, our expertise ensures you get the right equipment for precise, reliable results. Contact our experts today to discuss your specific needs and how we can support your goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- CVD Diamond for Thermal Management Applications
- High Performance Laboratory Freeze Dryer
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















