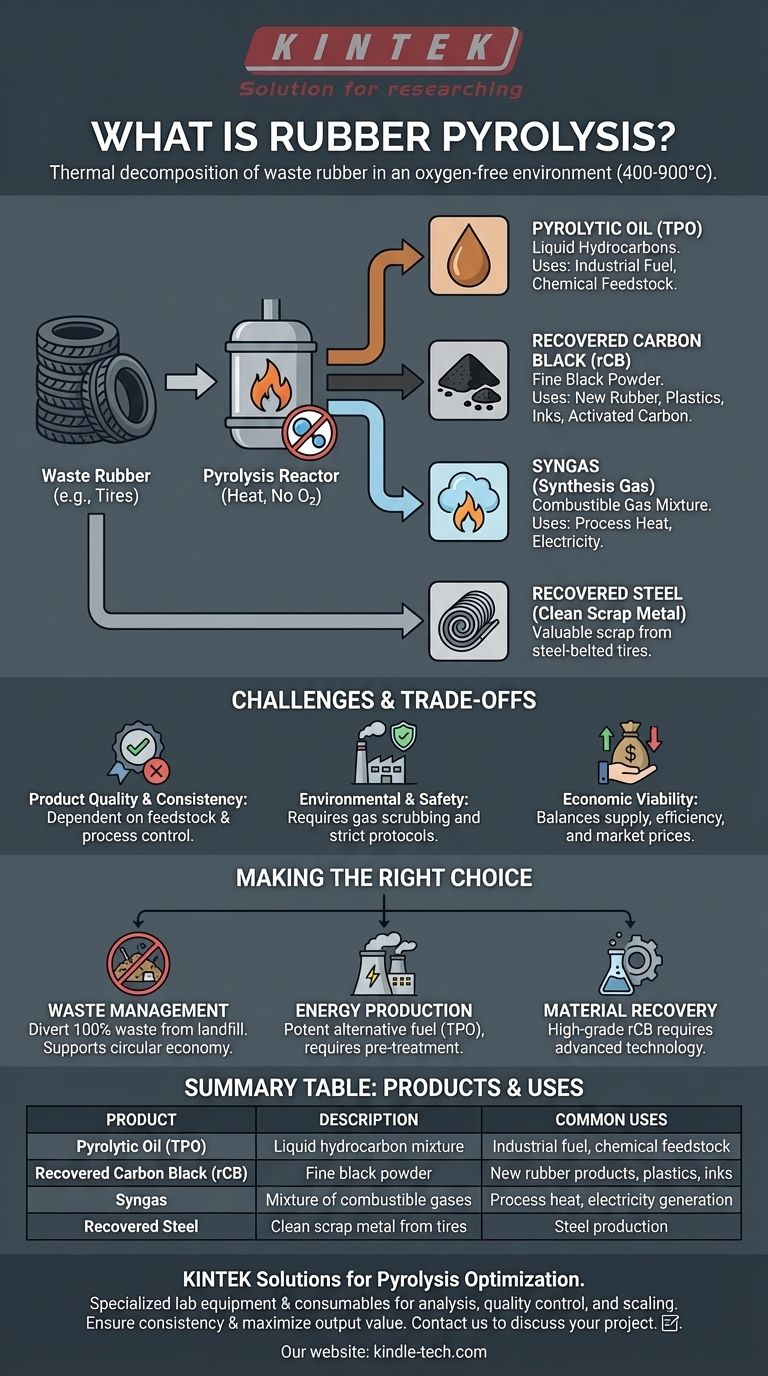
In simple terms, rubber pyrolysis is the process of breaking down waste rubber, such as end-of-life tires, using high heat in an environment without oxygen. This thermal decomposition process, typically occurring between 400-900°C, avoids combustion and instead chemically reverts the complex rubber polymers back into their core components: a liquid oil, a solid char, and a synthetic gas.
Rubber pyrolysis is not simply waste disposal; it is a chemical recycling method that transforms a problematic waste stream into a portfolio of potentially valuable industrial products. However, its environmental and economic success is entirely dependent on the quality of the technology and the consistency of its outputs.

The Core Mechanism: Deconstructing Rubber without Fire
Pyrolysis is fundamentally about controlled deconstruction. By manipulating heat and pressure in a reactor vessel, we can reverse the manufacturing process that created the rubber in the first place, breaking it down into smaller, more useful molecules.
The Role of Heat and an Oxygen-Free Environment
The key to pyrolysis is the absence of oxygen. When you heat rubber with oxygen present, it simply burns, releasing energy and creating ash and harmful emissions. By removing oxygen, we prevent combustion and force the long, cross-linked polymer chains within the rubber to break apart (a process called thermochemical decomposition).
The Three Primary Outputs
This decomposition results in three distinct product streams, each with its own characteristics and potential uses. The exact yield and quality of each product depend heavily on the feedstock (e.g., car tires vs. industrial rubber) and the specific process conditions like temperature and heating rate.
Analyzing the Outputs: From Waste Tire to Commodity
The goal of pyrolysis is to create marketable products from materials that would otherwise end up in a landfill. The value of the entire operation hinges on the quality and marketability of these outputs.
Pyrolytic Oil (TPO)
This liquid fraction is often considered the primary financial driver of rubber pyrolysis. It is a complex mixture of hydrocarbons, sometimes referred to as Tire-Derived Oil (TDO). While it has a high calorific value similar to industrial fuel oil, it contains sulfur and other impurities that often require further refining before it can be used as a clean-burning fuel or as a feedstock for the chemical industry.
Recovered Carbon Black (rCB)
This is the solid residue left after pyrolysis, a fine black powder. Its properties are influenced by the original carbon black used to manufacture the tire. High-quality rCB can be used as a reinforcing agent in new rubber products, as a pigment in plastics and inks, or be upgraded into activated carbon for filtration applications. However, lower-quality or contaminated rCB has limited use beyond being a low-grade solid fuel.
Syngas (Synthesis Gas)
This non-condensable gas fraction is a mixture of combustible gases like hydrogen, methane, and carbon monoxide. While it can be cleaned and used to generate electricity, its most common application is being recycled back into the system to provide the heat needed to run the pyrolysis reactor, making the process more energy self-sufficient.
Recovered Steel
When pyrolyzing steel-belted tires, the steel wire is recovered as a clean, high-quality scrap metal. This adds a consistent and valuable revenue stream to the overall business model.
Understanding the Trade-offs and Challenges
While pyrolysis presents a compelling solution to the global tire waste problem, it is not a perfect or simple technology. Its implementation comes with significant technical and economic hurdles.
Product Quality and Consistency
The biggest challenge is ensuring the quality and consistency of the pyrolytic oil and recovered carbon black. Variations in feedstock or poor process control can lead to outputs that fail to meet market specifications, drastically reducing their value and jeopardizing the plant's profitability.
Environmental and Safety Concerns
If not managed properly, pyrolysis plants can pose environmental risks. The process can release sulfur compounds and other pollutants, requiring sophisticated and expensive gas scrubbing systems. Handling large quantities of flammable oil and gas also demands stringent safety protocols to prevent accidents.
Economic Viability
The business case for a pyrolysis plant is a delicate balance. It depends on securing a low-cost, steady supply of waste tires, high operational efficiency, and stable market prices for the oil, carbon, and steel. Fluctuations in commodity prices can quickly change the profitability of an operation.
Making the Right Choice for Your Goal
Rubber pyrolysis is a technology with multiple potential benefits, but its application must align with a clear objective.
- If your primary focus is waste management: Pyrolysis is an excellent method for diverting 100% of a tire from landfill, supporting circular economy principles by turning waste into resources.
- If your primary focus is energy production: Pyrolytic oil can serve as a potent alternative fuel, but you must account for the necessity and cost of pre-treatment or refining to meet emissions standards.
- If your primary focus is material recovery: Success depends on producing high-grade recovered carbon black (rCB) that can compete with virgin materials, which requires advanced technology and strict process control.
Ultimately, rubber pyrolysis represents a powerful tool for valorizing waste, but its success is a matter of precise engineering, rigorous quality control, and a clear understanding of market dynamics.
Summary Table:
| Product | Description | Common Uses |
|---|---|---|
| Pyrolytic Oil (TPO) | Liquid hydrocarbon mixture | Industrial fuel, chemical feedstock |
| Recovered Carbon Black (rCB) | Fine black powder | New rubber products, plastics, inks |
| Syngas | Mixture of combustible gases | Process heat, electricity generation |
| Recovered Steel | Clean scrap metal from tires | Steel production |
Ready to turn your waste management challenge into a profitable resource recovery operation?
KINTEK specializes in advanced laboratory equipment and consumables for analyzing and optimizing pyrolysis processes. Whether you are researching feedstock, testing product quality, or scaling up your operation, our precise tools help you ensure consistency and maximize the value of your outputs—like high-grade recovered carbon black and clean pyrolytic oil.
Contact us today using the form below to discuss how our solutions can support your pyrolysis project, from initial research to final product quality control.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- What are the elements of blown film? Master the Key Components for High-Quality Film Production
- What are the different types of internal mixers? Choose Between Tangential & Intermeshing Rotors
- What are the disadvantages of blown film extrusion? Overcoming Precision and Speed Limitations
- What is twin screw extrusion? Achieve Superior Mixing and Uniform Product Quality
- What is the difference between Banbury and internal mixer? Understanding Rotor Design for Better Mixing
- What is injection molding in simple words? A Simple Guide to Mass-Producing Parts
- What are the three types of rolling mills? A Guide to Boosting Your Metal Production Efficiency
- What is the manufacturing process of rubber molding? Injection, Compression, or Transfer Molding?



















