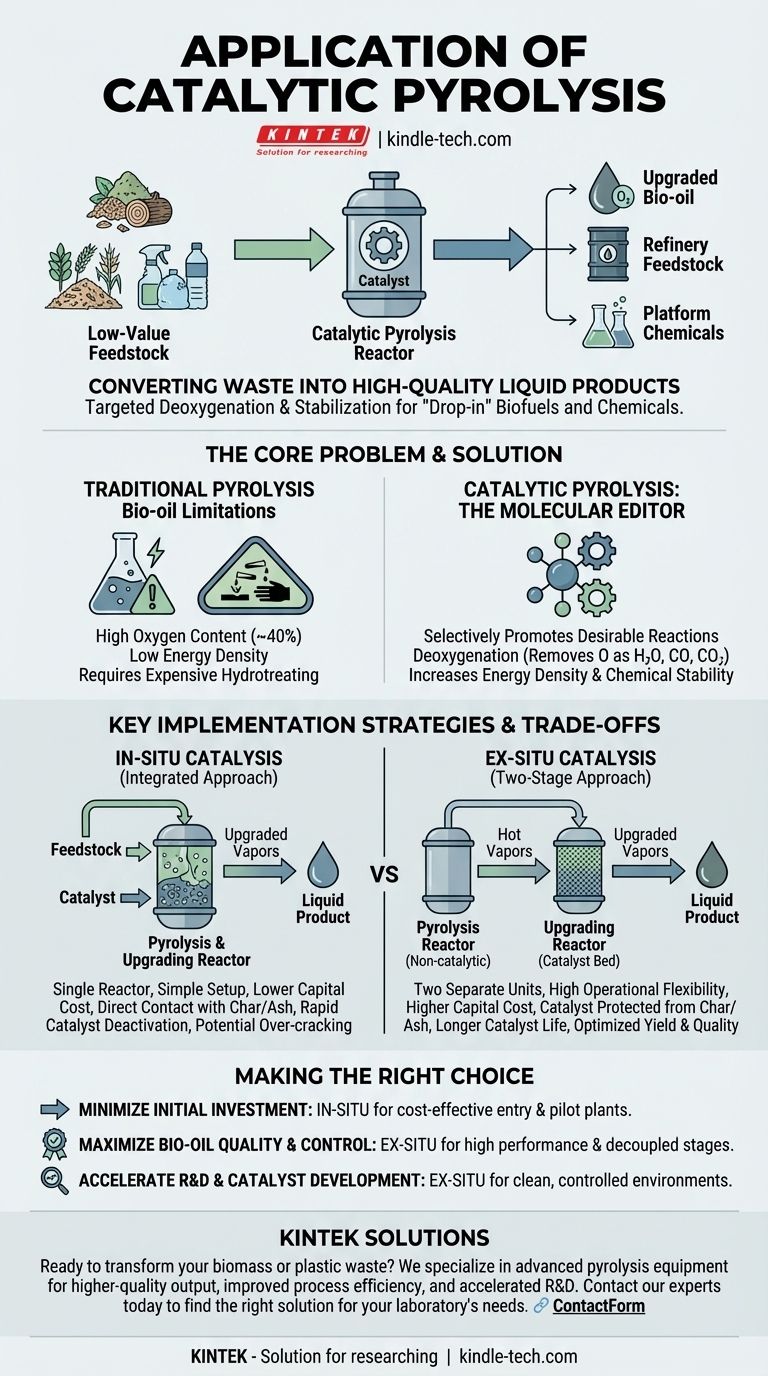
The primary application of catalytic pyrolysis is to convert low-value biomass or plastic waste into a significantly higher-quality liquid product, known as upgraded bio-oil. This process aims to deoxygenate and stabilize the oil directly during production, making it more suitable for use as a "drop-in" biofuel, a refinery feedstock, or a source for valuable platform chemicals, thereby overcoming the major drawbacks of non-catalytic pyrolysis.
The core purpose of catalytic pyrolysis is not just to convert waste into liquid, but to chemically upgrade the quality of that liquid in real-time. It aims to produce a more refined, stable, and valuable product, reducing the need for extensive and costly downstream processing.

The Core Problem Catalytic Pyrolysis Solves
To understand the application of catalytic pyrolysis, we must first understand the limitations of traditional pyrolysis.
Limitations of Traditional Pyrolysis Bio-oil
Standard pyrolysis effectively liquefies materials like wood, agricultural residues, or plastic waste. However, the resulting bio-oil is highly acidic, chemically unstable, and contains a large amount of oxygen (up to 40% by weight).
This high oxygen content makes the oil corrosive, gives it a low energy density, and prevents it from being mixed with conventional fossil fuels. It requires significant, expensive hydrotreating before it can be used in a standard refinery.
The Role of the Catalyst: A Molecular Editor
A catalyst introduced into the pyrolysis process acts as a molecular editor. Its function is to selectively promote desirable chemical reactions as the pyrolysis vapors are formed.
These reactions primarily involve deoxygenation, where oxygen atoms are removed from the vapor molecules in the form of H₂O, CO, and CO₂. This simultaneously increases the oil's energy density and chemical stability, creating a more hydrocarbon-like final product.
Key Implementation Strategies
The method of introducing the catalyst fundamentally changes the process design, cost, and outcome. The choice between these strategies is central to any practical application.
In-Situ Catalysis: The Integrated Approach
In this method, the catalyst is mixed directly with the feedstock (e.g., biomass) inside a single pyrolysis reactor. This is the in-situ configuration.
The primary advantage is its simplicity and lower initial capital cost, as it only requires one main reactor vessel.
Ex-Situ Catalysis: The Two-Stage Approach
In an ex-situ configuration, the process is split into two separate units. First, the feedstock is pyrolyzed in a non-catalytic reactor. The resulting hot vapors are then immediately passed into a second, separate reactor containing the catalyst bed for upgrading.
This two-stage approach provides significantly more control over the entire process, allowing for the optimization of both the pyrolysis and the catalytic upgrading steps independently.
Understanding the Trade-offs: In-Situ vs. Ex-Situ
Choosing a strategy involves balancing cost, performance, and operational complexity. There is no single best answer; the optimal choice depends entirely on the project's goals.
Capital Cost vs. Operational Flexibility
In-situ systems are cheaper to build due to the single-reactor design. This makes them attractive for smaller-scale operations or initial pilot plants.
Ex-situ systems have a higher capital cost but offer far greater operational flexibility. You can control the temperature and residence time in each stage independently to maximize both liquid yield and upgrading quality.
Catalyst Performance and Lifespan
This is a critical distinction. In in-situ pyrolysis, the catalyst is directly exposed to char and inorganic ash from the biomass. This leads to rapid deactivation through coking and poisoning, reducing its effectiveness and lifespan.
The ex-situ approach protects the catalyst. Because only hot vapors enter the second reactor, the catalyst is not contaminated by char or ash. This allows for a longer operational life, easier regeneration, and the potential to use more sophisticated (and expensive) catalysts that would be unviable in an in-situ setup.
Bio-Oil Yield vs. Quality
The intimate contact in in-situ systems can sometimes lead to over-cracking, where desirable liquid molecules are broken down into less valuable non-condensable gases, thus reducing the final oil yield.
With ex-situ systems, operators can fine-tune conditions in the upgrading reactor to achieve the desired level of deoxygenation without excessively reducing the overall liquid yield.
Making the Right Choice for Your Goal
The decision to use catalytic pyrolysis, and how to implement it, must align with your primary objective.
- If your primary focus is minimizing initial investment: An in-situ design is the most direct and cost-effective path to producing an upgraded bio-oil, accepting the trade-offs of lower catalyst life and process control.
- If your primary focus is maximizing bio-oil quality and process control: An ex-situ configuration is superior, as it decouples pyrolysis from upgrading and protects the catalyst, enabling higher performance and stability.
- If your primary focus is research and catalyst development: An ex-situ system is essential, as it provides the clean, controlled environment needed to accurately measure catalyst performance without interference from the feedstock.
Ultimately, applying catalytic pyrolysis is a strategic decision that shifts the goal from simple waste liquefaction to the targeted production of advanced biofuels and chemicals.
Summary Table:
| Feature | In-Situ Catalysis | Ex-Situ Catalysis |
|---|---|---|
| Setup | Single reactor (catalyst mixed with feedstock) | Two-stage process (separate pyrolysis & upgrading reactors) |
| Capital Cost | Lower initial investment | Higher initial investment |
| Process Control | Limited, integrated process | High, independent optimization of each stage |
| Catalyst Life | Shorter (exposed to char/ash) | Longer (protected from contaminants) |
| Best For | Cost-effective entry, pilot plants | Maximizing oil quality, research, large-scale operations |
Ready to transform your biomass or plastic waste into a valuable resource?
At KINTEK, we specialize in advanced laboratory equipment for pyrolysis and catalytic processes. Whether you are developing new catalysts, optimizing yields, or scaling up production, our solutions provide the precision and control you need to succeed.
We help our customers in the bioenergy and chemical sectors achieve:
- Higher-Quality Output: Produce stable, deoxygenated bio-oil ready for use as a biofuel or chemical feedstock.
- Improved Process Efficiency: Fine-tune pyrolysis and catalytic upgrading parameters for maximum yield and catalyst lifespan.
- Accelerated R&D: Access reliable equipment for critical research and development.
Let's discuss your project goals. Contact our experts today to find the right catalytic pyrolysis solution for your laboratory's needs.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas