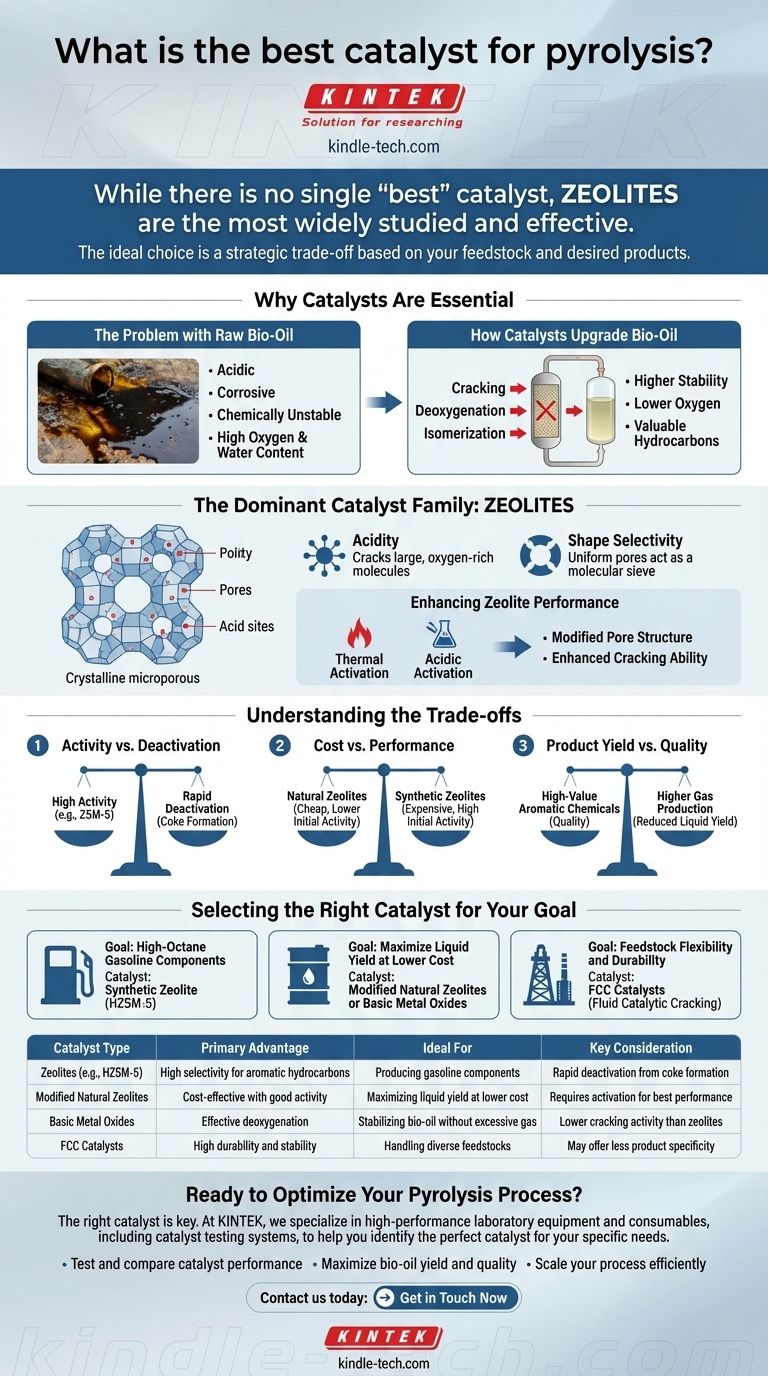
While there is no single "best" catalyst for all pyrolysis applications, the most widely studied and effective class of materials are zeolites. These catalysts are favored for their ability to significantly upgrade pyrolysis vapors into higher-quality liquid fuels and valuable chemicals. Their performance, however, depends heavily on the specific type of zeolite used and how it is modified for the task.
The ideal pyrolysis catalyst is not a single substance, but rather a strategic choice based on your specific feedstock and desired product. The decision hinges on a trade-off between catalytic activity, product selectivity, operational stability, and cost.

Why Catalysts Are Essential in Pyrolysis
Pyrolysis, the thermal decomposition of organic material without oxygen, produces a mix of solid char, non-condensable gases, and a liquid known as bio-oil. A catalyst is introduced to improve this process in several critical ways.
The Problem with Raw Bio-Oil
Raw bio-oil is not an ideal fuel. It is acidic, corrosive, chemically unstable, and has a high oxygen and water content, which lowers its energy density. Using it directly in engines or refineries is often impossible without significant upgrading.
How Catalysts Upgrade Bio-Oil
A catalyst's primary job is to steer the chemical reactions during pyrolysis toward more desirable outcomes. It provides an active surface that promotes cracking, deoxygenation, and isomerization reactions, effectively upgrading the vapors before they condense into bio-oil. This results in a final liquid product with higher stability, lower oxygen content, and a greater concentration of valuable hydrocarbons.
The Dominant Catalyst Family: Zeolites
Zeolites are the front-runners in catalytic pyrolysis due to their unique structural and chemical properties. They are crystalline microporous materials made of silicon, aluminum, and oxygen.
What Makes Zeolites Effective
The power of zeolites comes from two key features: acidity and shape selectivity.
Their internal structure contains acid sites that are highly effective at cracking large, oxygen-rich molecules into smaller, more valuable hydrocarbons. At the same time, the uniform, molecular-sized pores act as a "molecular sieve," controlling which molecules can enter and which products can be formed, guiding the process toward specific outputs like aromatic compounds found in gasoline.
Enhancing Zeolite Performance
Even within the zeolite family, performance can be fine-tuned. As noted in research, simple natural zeolites (NZ) can be made more effective through activation methods.
Thermal activation (heating) and acidic activation (washing with acid) can modify the pore structure and the number of active acid sites on the catalyst. These treatments enhance its ability to crack feedstock molecules, improving the quality and yield of the final bio-fuel.
Understanding the Trade-offs
Choosing a catalyst is an exercise in balancing competing priorities. There is no perfect solution, only the best compromise for a specific goal.
Activity vs. Deactivation
Highly active catalysts, particularly synthetic zeolites like ZSM-5, are excellent at producing high-quality aromatic hydrocarbons. However, this high activity often leads to rapid deactivation as coke (a solid carbon byproduct) forms on the catalyst surface, blocking the active sites.
Cost vs. Performance
Natural zeolites are abundant and significantly cheaper than their highly engineered synthetic counterparts. While their catalytic performance may be lower initially, modification techniques can make them a highly cost-effective option, especially for large-scale operations.
Product Yield vs. Quality
Optimizing for one output often comes at the expense of another. A catalyst that excels at producing high-value aromatic chemicals may do so by converting a larger fraction of the bio-oil into gas, thereby reducing the overall liquid yield.
Selecting the Right Catalyst for Your Goal
Your choice of catalyst should be dictated by your primary objective.
- If your primary focus is producing high-octane gasoline components: A synthetic zeolite like HZSM-5 is the established benchmark due to its exceptional shape selectivity for producing valuable aromatic hydrocarbons.
- If your primary focus is maximizing liquid yield at a lower cost: Modified natural zeolites or basic metal oxides (like calcium or magnesium oxide) are excellent choices for deoxygenating bio-oil without over-cracking it into gases.
- If your primary focus is feedstock flexibility and durability: Fluid Catalytic Cracking (FCC) catalysts, borrowed from the petroleum industry, offer a robust and stable option designed to handle diverse materials and resist deactivation.
Ultimately, selecting a catalyst is the most critical decision in designing an efficient and economically viable pyrolysis process.
Summary Table:
| Catalyst Type | Primary Advantage | Ideal For | Key Consideration |
|---|---|---|---|
| Zeolites (e.g., HZSM-5) | High selectivity for aromatic hydrocarbons | Producing gasoline components | Rapid deactivation from coke formation |
| Modified Natural Zeolites | Cost-effective with good activity | Maximizing liquid yield at lower cost | Requires activation (thermal/acidic) for best performance |
| Basic Metal Oxides | Effective deoxygenation | Stabilizing bio-oil without excessive gas production | Lower cracking activity than zeolites |
| FCC Catalysts | High durability and stability | Handling diverse feedstocks in large-scale operations | May offer less product specificity |
Ready to Optimize Your Pyrolysis Process?
The right catalyst is the key to transforming your pyrolysis output. At KINTEK, we specialize in providing high-performance laboratory equipment and consumables, including catalyst testing systems, to help you identify the perfect catalyst for your specific biomass feedstock and target products.
Our experts can help you:
- Test and compare catalyst performance for your unique application.
- Maximize bio-oil yield and quality with precision-controlled pyrolysis reactors.
- Scale your process efficiently from lab bench to pilot plant.
Contact us today to discuss your project and discover how KINTEK's solutions can accelerate your bio-fuel and chemical production. ➡️ Get in Touch Now
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What is the substrate temperature in sputtering? Master Film Quality with Precise Thermal Control
- What is the standard for Aluminium heat treatment? Master the Temper Designation System for Optimal Properties
- Why is precise temperature and pressure control necessary for combustible cartridge cases? Ensure Structural Integrity
- What is the HIP process of metal? Achieve Perfect Density for Critical Components
- What is gold coating SEM for? Prevent Charging & Get Clearer SEM Images
- What are CBD distillates? Discover the Key Differences Between Full, Broad & Isolate
- How does a high-power ultrasonic homogenizer assist in the preparation of organic-inorganic nanocomposites?



















