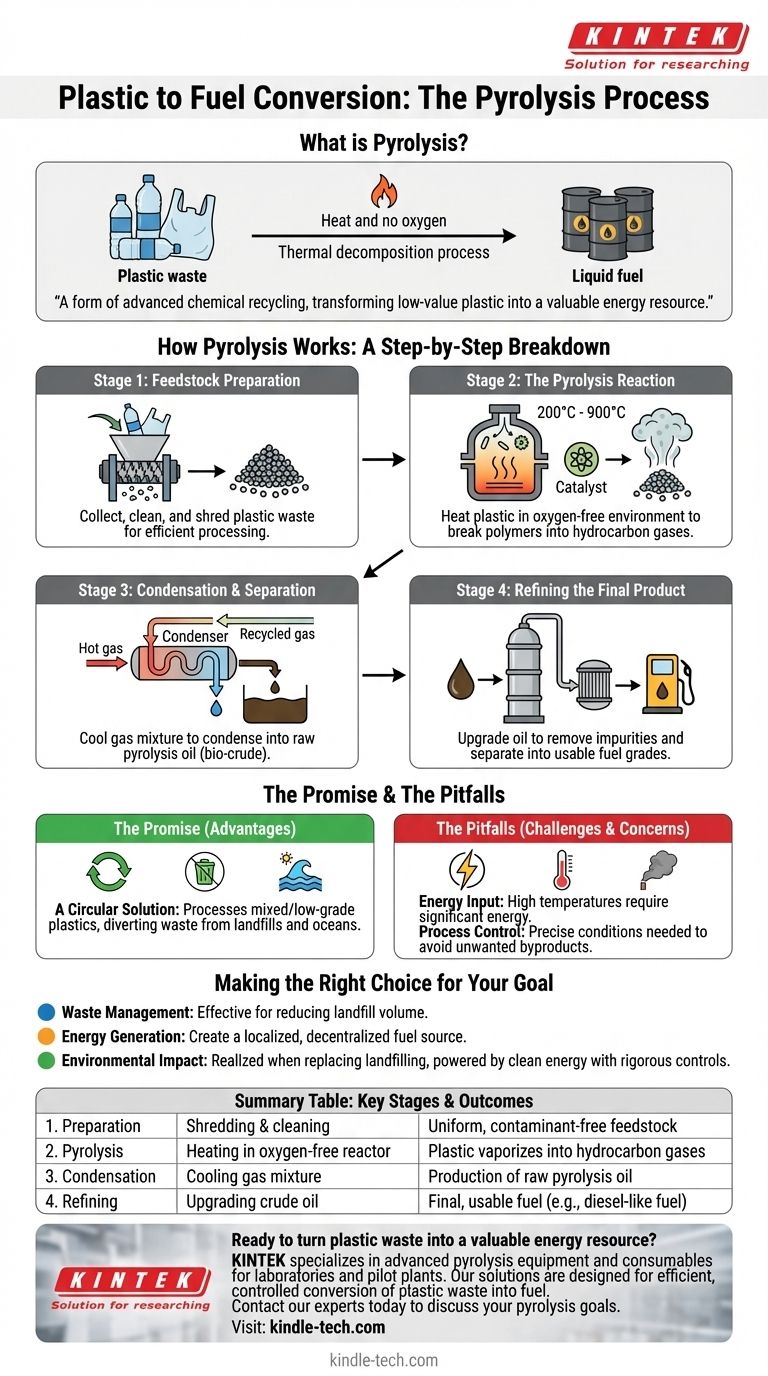
In essence, the conversion of plastic to fuel by pyrolysis is a thermochemical decomposition process. It uses intense heat in an oxygen-free environment to break down long-chain plastic polymers into smaller, simpler hydrocarbon molecules, which can then be refined into a liquid fuel similar to diesel or gasoline.
At its core, pyrolysis is a form of advanced chemical recycling. It reverts plastic waste back to its fundamental components, transforming a low-value, environmentally persistent pollutant into a valuable energy resource.

How Pyrolysis Works: A Step-by-Step Breakdown
To understand the potential of pyrolysis, it's essential to grasp the distinct stages of the process, from waste collection to final fuel product.
Stage 1: Feedstock Preparation
Before any thermal process begins, the raw plastic waste must be prepared. This involves collecting the waste, cleaning it to remove non-plastic contaminants like dirt or metal, and then shredding or grinding it into a uniform, smaller size.
This pre-treatment is critical for ensuring an efficient and consistent reaction inside the pyrolysis chamber.
Stage 2: The Pyrolysis Reaction
The prepared plastic is loaded into a sealed reactor. The chamber is purged of oxygen and heated to extremely high temperatures, typically between 200°C and 900°C.
This intense heat, often aided by a catalyst, provides the energy to break the strong chemical bonds holding the long plastic polymer chains together. Because there is no oxygen, the plastic doesn't burn; it vaporizes into a mixture of hydrocarbon gases.
Stage 3: Condensation and Separation
The hot gas mixture is directed out of the reactor and into a condensation system. As the gas cools, it condenses back into a liquid.
This raw liquid product is often called pyrolysis oil or "bio-crude." Any non-condensable gases are typically recycled to help fuel the reactor, improving the system's overall energy efficiency.
Stage 4: Refining the Final Product
The raw pyrolysis oil is then collected. It often requires further refining or upgrading to remove remaining impurities and to separate it into different fuel grades, such as a diesel-like fuel, which can then be used in engines or generators.
The Promise and The Pitfalls
While pyrolysis presents a compelling solution to plastic waste, a balanced perspective requires understanding its advantages and inherent challenges.
The Advantage: A Circular Solution
Pyrolysis is a powerful tool for chemical recycling. It can process mixed, contaminated, and lower-grade plastics that are unsuitable for traditional mechanical recycling, diverting massive amounts of waste from landfills and oceans.
The Challenge: Energy Input
The process is energy-intensive. Reaching and maintaining the high temperatures required for pyrolysis consumes a significant amount of energy, which can impact the net energy gain and overall environmental footprint of a facility.
The Concern: Process Control
If the process is not managed perfectly, it has the potential to create unwanted byproducts or emissions. The quality of the final fuel and the safety of the operation depend heavily on precise control over temperature, pressure, and feedstock purity.
Making the Right Choice for Your Goal
Understanding pyrolysis allows you to evaluate its role based on specific objectives.
- If your primary focus is waste management: Pyrolysis is a highly effective method for reducing the volume of non-recyclable plastic waste destined for landfills.
- If your primary focus is energy generation: It offers a pathway to create a localized, decentralized fuel source from a readily available waste stream.
- If your primary focus is environmental impact: The technology's true benefit is realized when it replaces landfilling and is powered by clean energy, with rigorous controls on all outputs.
Ultimately, viewing pyrolysis as a sophisticated waste-valorization technology is the most accurate way to assess its place in a circular economy.
Summary Table:
| Stage | Process | Key Outcome |
|---|---|---|
| 1. Preparation | Shredding & cleaning plastic waste | Uniform, contaminant-free feedstock |
| 2. Pyrolysis | Heating in an oxygen-free reactor (200-900°C) | Plastic vaporizes into hydrocarbon gases |
| 3. Condensation | Cooling the gas mixture | Production of raw pyrolysis oil |
| 4. Refining | Upgrading the crude oil | Final, usable fuel (e.g., diesel-like fuel) |
Ready to turn plastic waste into a valuable energy resource?
KINTEK specializes in advanced pyrolysis equipment and consumables for laboratories and pilot plants. Our solutions are designed for efficient, controlled conversion of plastic waste into fuel, helping you advance your waste management and energy recovery projects.
Contact our experts today to discuss how our reliable lab equipment can support your pyrolysis research and development goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process



















