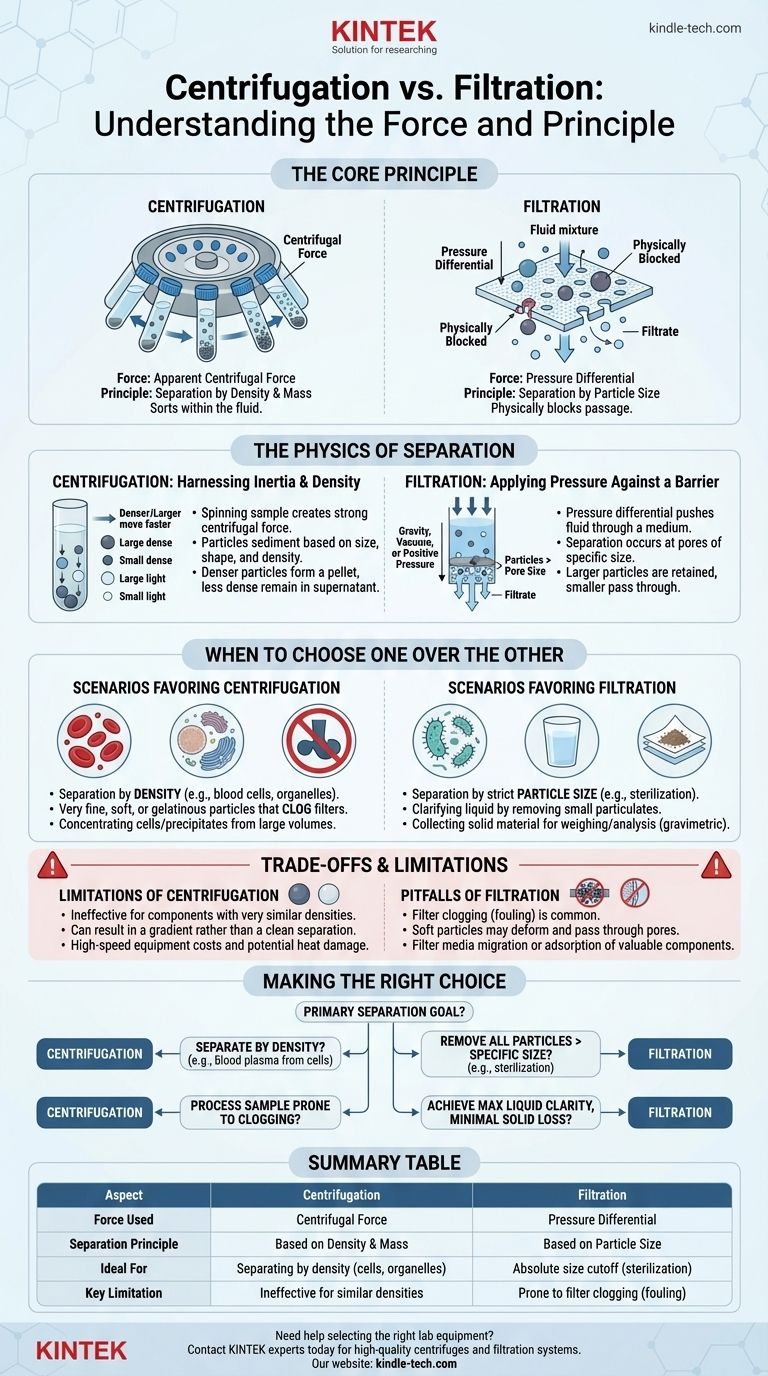
At its core, the difference is one of principle: centrifugation uses an apparent centrifugal force to separate components based on their density and mass, while filtration uses a physical pressure differential to force a fluid through a porous barrier, separating particles based on size. Centrifugation sorts particles within the fluid, whereas filtration physically blocks them from passing through.
The crucial distinction is not the type of force, but the mechanism of separation. Centrifugation separates based on the inherent physical properties of the particles themselves (like density), while filtration separates based on an external physical barrier (the filter's pore size).

The Physics of Separation: A Tale of Two Forces
Understanding which method to use requires a clear grasp of the fundamental forces and principles each technique employs.
Centrifugation: Harnessing Inertia and Density
Centrifugation works by spinning a sample at high velocity. This rotation creates a strong centrifugal force, an apparent outward force that acts on all particles within the sample.
This force causes particles to sediment, or move away from the center of rotation. The rate of sedimentation is not uniform; it depends heavily on a particle's size, shape, and density relative to the surrounding fluid.
Denser or larger particles move outward faster, forming a pellet at the bottom of the tube, while less dense components remain suspended in the liquid (supernatant).
Filtration: Applying Pressure Against a Barrier
Filtration relies on a simple mechanical principle: a pressure differential. This force pushes the bulk fluid through a filter medium.
This pressure can be generated by gravity, a vacuum applied downstream of the filter, or positive pressure applied upstream. The force acts on the entire fluid, compelling it to move.
Separation occurs because the filter contains pores of a specific size. Any particles in the fluid that are larger than the pores are physically blocked and retained, while the fluid (filtrate) and smaller dissolved components pass through.
When to Choose One Over the Other
The choice between these methods is dictated entirely by the nature of your sample and your desired outcome.
Scenarios Favoring Centrifugation
You should choose centrifugation when separation needs to be based on density. This is critical for separating components that might be of similar size but have different densities, like separating blood cells or subcellular organelles.
It is also the superior method for handling samples with very fine, soft, or gelatinous particles. These types of particles would quickly clog a filter, but can be effectively pelleted with sufficient centrifugal force.
Finally, centrifugation is ideal for concentrating cells or precipitates from a large volume of liquid into a small, dense pellet for subsequent analysis.
Scenarios Favoring Filtration
Filtration is the go-to method when separation must be based strictly on particle size. Its most common application is sterilization, where you need to remove all bacteria (e.g., 0.22 µm) from a heat-sensitive solution.
It is also highly effective for clarifying a liquid by removing a small amount of solid particulate contamination. The result is a particle-free filtrate.
Furthermore, if the goal is to collect the solid material itself on a clean surface for weighing or analysis (gravimetric analysis), filtration is the correct technique.
Understanding the Trade-offs and Limitations
Neither technique is perfect. Being aware of their inherent limitations is key to avoiding failed experiments and poor results.
The Limitations of Centrifugation
The primary drawback of centrifugation is its ineffectiveness when components have very similar densities. Achieving a clean separation in such cases can be impossible.
Separation is often a matter of degree, resulting in a gradient rather than a clean, absolute division. This can lead to cross-contamination between the pellet and the supernatant.
High-speed centrifuges are also significant investments, require meticulous balancing to operate safely, and can generate heat that might damage biologically active samples.
The Pitfalls of Filtration
The most common failure in filtration is filter clogging, also known as fouling. As retained particles accumulate, they block the pores, drastically reducing the flow rate and potentially causing the filter to rupture.
Softer, deformable particles can be squeezed through filter pores that are technically smaller than the particle's resting diameter, leading to incomplete separation.
Finally, the filter itself can be a source of problems. It may shed fibers into your filtrate (media migration) or adsorb valuable proteins or small molecules from your sample, reducing your yield.
Making the Right Choice for Your Separation Goal
Base your decision on the physical properties of your sample and the specific outcome you need to achieve.
- If your primary focus is separating components by density (e.g., blood plasma from cells): Centrifugation is the correct and most effective tool.
- If your primary focus is removing all particles above a specific size (e.g., sterilizing a solution): Filtration provides an absolute size cutoff that centrifugation cannot guarantee.
- If your primary focus is processing a sample prone to clogging (e.g., cell lysate): Centrifugation avoids the clogging issues inherent to filtration and is often more reliable.
- If your primary focus is achieving maximum liquid clarity with minimal solid loss: Filtration is generally superior for producing a particle-free filtrate.
By understanding the fundamental forces at play, you can confidently select the most effective separation method for your specific objective.
Summary Table:
| Aspect | Centrifugation | Filtration |
|---|---|---|
| Force Used | Centrifugal Force | Pressure Differential |
| Separation Principle | Based on Density & Mass | Based on Particle Size |
| Ideal For | Separating by density (e.g., cells, organelles) | Absolute size cutoff (e.g., sterilization) |
| Key Limitation | Ineffective for similar densities | Prone to filter clogging (fouling) |
Need help selecting the right lab equipment for your separation tasks? At KINTEK, we specialize in providing high-quality laboratory equipment and consumables, including centrifuges and filtration systems, to meet your specific research needs. Our experts can help you choose the perfect solution to enhance your lab's efficiency and accuracy. Contact us today to discuss your requirements and discover how KINTEK can support your laboratory's success!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tubes
- High Shear Homogenizer for Pharmaceutical and Cosmetic Applications
- Manual Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Automatic Laboratory Heat Press Machine
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
People Also Ask
- What role does an ultrasonic homogenizer play in the preparation of nickel nanoparticle colloids? Enhance Dispersion
- How does a sputter coater work? A Guide to Atomic-Level Thin Film Deposition
- What happens in sputtering? A Step-by-Step Guide to Thin Film Deposition
- Where are evaporators used in food industry? Concentrate Products & Reduce Costs
- What are the working principles of electric arc furnace? Harnessing the Power of an Electric Arc for High-Temperature Melting
- Why is precise temperature-controlled heating equipment required for chitosan synthesis? Ensure High-Quality Deacetylation
- What is the number 1 rule of soldering? Master the Heat for Strong, Reliable Connections
- What is the simple explanation of pyrolysis? A Guide to Waste-to-Energy Conversion



















