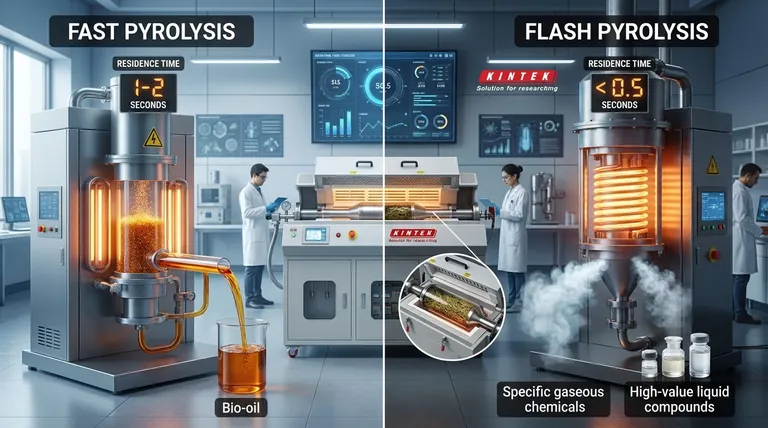
The fundamental difference between flash pyrolysis and fast pyrolysis is the timescale of the reaction. While both involve very rapid heating, flash pyrolysis operates on an even shorter vapor residence time—typically less than half a second—with extremely high heating rates. Fast pyrolysis is slightly slower, with residence times around 1-2 seconds. This subtle distinction in speed is critical as it directly controls the final product distribution.
While the terms are sometimes used interchangeably in general discussion, in a technical context, the distinction is crucial. Fast pyrolysis is engineered to maximize liquid bio-oil yield, while the more extreme conditions of flash pyrolysis are often used to target specific, high-value chemical compounds by preventing them from degrading in secondary reactions.
The Core Principle: A Race Against Time
Pyrolysis is the thermal decomposition of organic material, like biomass, in the absence of oxygen. The process breaks down large complex molecules (cellulose, lignin) into smaller, more valuable ones. The key to understanding the different types of pyrolysis is to see them as a spectrum of reaction speeds.
Slow Pyrolysis: The Baseline
Slow pyrolysis uses low heating rates over long periods (minutes to hours). This gives the molecules ample time to react and re-condense into stable carbon structures.
The primary product of this unhurried process is biochar, a solid, carbon-rich material.
Fast Pyrolysis: Maximizing Liquid Yield
Fast pyrolysis drastically increases the heating rate and shortens the time the hot vapors spend in the reactor (the "vapor residence time") to about 1-2 seconds.
The goal is to rapidly break down the biomass and then immediately quench the vapors, effectively "freezing" the reaction products in their liquid state before they can break down further. This process is optimized to produce the maximum yield of bio-oil.
Flash Pyrolysis: Targeting Specific Chemicals
Flash pyrolysis pushes the parameters to their practical limits. It involves extremely high heating rates and a vapor residence time of less than 0.5 seconds, often in the millisecond range.
This ultra-short duration is designed to minimize secondary reactions. As soon as the initial valuable chemical compounds are formed, they are removed from the hot zone before they can crack into less valuable gases or repolymerize into char and tar.
Why This Time Difference is Critical
The speed of the process directly dictates the chemical pathways that are favored, which in turn determines the final product slate.
Impact on Product Yield and Quality
A longer residence time allows for secondary reactions to occur. The initial liquid products (primary vapors) can crack into lighter gases or re-polymerize into char and heavy tars.
Fast pyrolysis strikes a balance, allowing for high bio-oil yield (up to 75% by weight) before significant degradation occurs. Flash pyrolysis is an attempt to capture only those primary vapors, which can result in higher yields of specific valuable chemicals like levoglucosan or olefins.
The Role of Reactor Design
Achieving these precise conditions requires specialized equipment. The reactor types you see mentioned, such as fluidized-bed and ablative reactors, are common for fast pyrolysis because they ensure the rapid and uniform heat transfer necessary to process biomass particles quickly.
Research-scale flash pyrolysis often employs even more specialized designs like entrained-flow or wire-mesh reactors to achieve the near-instantaneous heating and short residence times required.
Understanding the Trade-offs
Choosing a pyrolysis method is not just about speed; it's about balancing engineering complexity with the desired outcome and economic viability.
Engineering Complexity and Scale
The faster the process, the more difficult it is to control and scale up. Flash pyrolysis systems are often more complex and sensitive to operating conditions than fast or slow pyrolysis reactors, limiting their use in large-scale industrial applications.
Feedstock Requirements
Rapid heat transfer is only possible if the biomass particles are very small and dry. Both fast and flash pyrolysis require significant energy and cost for grinding and drying the feedstock before it can even enter the reactor.
Cost vs. Product Value
The decision ultimately comes down to economics. If your goal is to produce large volumes of bio-oil as a precursor to biofuel, the robust and scalable nature of fast pyrolysis is ideal. If you are trying to produce a specific high-value chemical for the pharmaceutical or specialty chemical industry, the added complexity and cost of flash pyrolysis may be justified.
Making the Right Choice for Your Goal
To select the correct process, you must first define your target product.
- If your primary focus is maximizing biochar yield for soil amendment or carbon sequestration, the long residence times of slow pyrolysis are the correct choice.
- If your primary focus is maximizing liquid bio-oil for biofuels or as a chemical feedstock, the balanced conditions of fast pyrolysis are the industry standard.
- If your primary focus is maximizing specific, high-value primary chemical compounds by avoiding secondary reactions, the extreme conditions of flash pyrolysis are required.
Ultimately, controlling the time and temperature of pyrolysis is how you control the chemistry and engineer the precise output you need.
Summary Table:
| Parameter | Fast Pyrolysis | Flash Pyrolysis |
|---|---|---|
| Vapor Residence Time | 1-2 seconds | < 0.5 seconds (often milliseconds) |
| Primary Goal | Maximize liquid bio-oil yield (up to 75%) | Target specific high-value chemicals |
| Key Characteristic | Rapid heating, immediate vapor quenching | Extremely rapid heating, minimal secondary reactions |
| Common Reactor Types | Fluidized-bed, ablative reactors | Entrained-flow, wire-mesh reactors |
| Ideal For | Biofuel production, chemical feedstocks | Pharmaceuticals, specialty chemicals |
Ready to engineer the perfect pyrolysis process for your biomass conversion goals?
At KINTEK, we specialize in advanced laboratory equipment for pyrolysis research and development. Whether you're scaling up bio-oil production or targeting high-value chemical compounds, our reactors and consumables are designed for precision, control, and reliability. Let our experts help you select the right system to maximize your product yield and achieve your specific outcomes.
Contact us today to discuss your project and discover how KINTEK can power your innovation!
Visual Guide

Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What are the two main configurations of ULT freezers? Upright vs. Chest for Your Lab's Needs
- What is the double sintering method? Achieve Maximum Density with Controlled Microstructure
- What is the mechanism of reactive sputtering? A Guide to Thin Film Deposition
- How is heat transferred in a furnace? Master Radiation, Convection & Conduction
- What is extruded graphite? Understanding Its Anisotropic Properties for Cost-Effective Solutions
- What is the effect of catalyst on pyrolysis? Control Reaction Pathways for Higher-Value Products
- What are the applications of RF sputtering? Enabling Advanced Thin-Film Deposition for Insulators
- Is pyrolysis oil a biofuel? Understanding Its Potential as a Renewable Energy Source



















