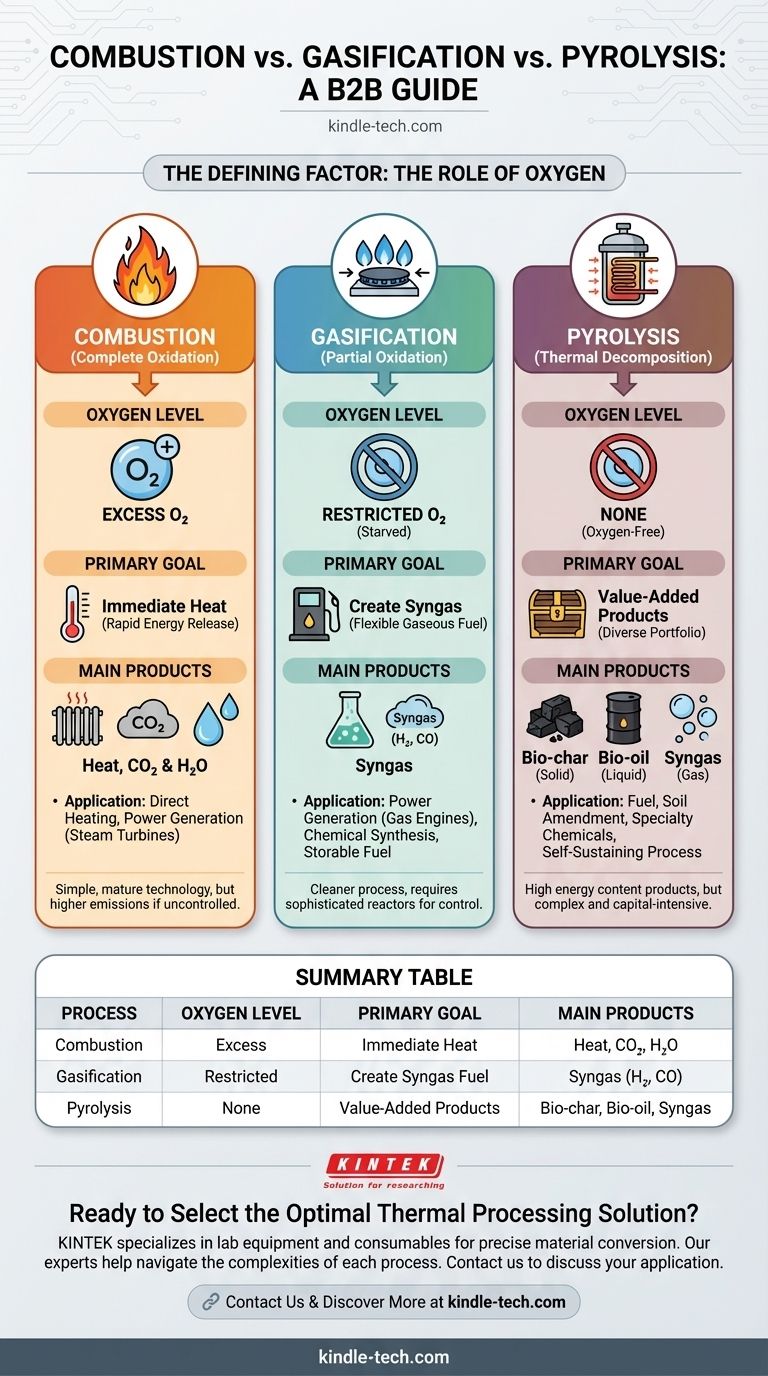
At their core, the difference between combustion, gasification, and pyrolysis is the amount of oxygen present during the process. Combustion is the complete burning of a material with a surplus of oxygen to generate heat. Gasification uses a restricted amount of oxygen to convert material into a combustible gas, while pyrolysis uses no oxygen at all, thermally decomposing material into a mix of solid, liquid, and gaseous products.
The choice between these three thermal processes is not about which is universally "better," but which is best suited for your end goal. The fundamental trade-off is between generating immediate heat (combustion) versus creating storable, value-added fuels and products (gasification and pyrolysis).

The Defining Factor: The Role of Oxygen
The presence or absence of oxygen dictates the entire chemical pathway and, consequently, the final output of each process. Think of oxygen as the key ingredient that determines the recipe.
Combustion: Complete Oxidation for Maximum Heat
Combustion is the most familiar process—it is simply burning. It involves reacting a fuel source with an excess of oxygen to achieve complete oxidation.
The primary goal of combustion is the rapid and total release of the material's chemical energy as heat. The main byproducts are typically carbon dioxide (CO₂) and water (H₂O).
Gasification: Partial Oxidation for Gaseous Fuel
Gasification deliberately starves the reaction of the oxygen it needs for full combustion. By supplying a restricted amount of oxygen, the organic material is only partially oxidized.
This process breaks down the material into a mixture of combustible gases known as syngas (synthesis gas), which is primarily composed of hydrogen (H₂) and carbon monoxide (CO). This syngas is a fuel in itself.
Pyrolysis: Thermal Decomposition Without Oxygen
Pyrolysis is the process of heating organic material to high temperatures in a completely oxygen-free environment. Without oxygen, the material cannot "burn."
Instead, the heat breaks the complex molecules down into simpler, smaller molecules. Because it's an endothermic process (it requires energy input), the resulting products retain a very high energy content.
Comparing the Outputs: What Each Process Creates
The difference in process chemistry directly leads to a different portfolio of products, each with distinct uses and economic value.
Combustion's Product: Immediate Heat
The only significant energy product from combustion is heat. This is ideal for applications where direct heating, boiling water to create steam, or driving a steam turbine for power is the immediate goal.
Gasification's Product: Syngas as a Flexible Fuel
Gasification's primary product, syngas, is a versatile energy carrier. It can be burned immediately in a gas engine to generate electricity, stored for later use, or serve as a chemical building block to synthesize liquid fuels and other valuable chemicals.
Pyrolysis's Products: A Portfolio of Valuables
Pyrolysis creates three distinct product streams:
- Bio-char: A stable, carbon-rich solid similar to charcoal. It can be used as a fuel, a soil amendment, or for carbon sequestration.
- Bio-oil (or Pyrolysis Oil): A dense, dark liquid that can be refined into transportation fuels or used to produce specialty chemicals.
- Syngas: A gaseous mixture that can be used to provide the energy needed to sustain the pyrolysis process itself, making it partially self-sufficient.
Understanding the Trade-offs
Choosing a technology requires an objective look at its efficiency, complexity, and environmental footprint.
Environmental Considerations
Combustion, especially when uncontrolled, can produce significant pollutants like nitrogen oxides (NOx), sulfur oxides (SOx), and particulate matter.
Gasification and pyrolysis are generally considered cleaner. By controlling the process and capturing the products, emissions are drastically reduced, and pollutants are often retained in the bio-char or syngas, where they can be managed more easily.
Process Complexity and Cost
Combustion is a mature, relatively simple technology. Building a furnace or boiler is straightforward and cost-effective.
Gasification and pyrolysis require more sophisticated reactors and precise control systems to manage temperature and oxygen levels. This increases the initial capital cost and operational complexity.
Making the Right Choice for Your Goal
Selecting the correct technology depends entirely on your strategic objective, whether it's simple waste disposal, energy generation, or creating high-value commodities.
- If your primary focus is immediate, on-site heat generation: Combustion is the simplest and most direct pathway to convert a fuel's energy into heat.
- If your primary focus is creating a flexible gaseous fuel for power generation or synthesis: Gasification provides an intermediate fuel (syngas) that can be stored, transported, and used in multiple ways.
- If your primary focus is maximizing value and creating a diverse product portfolio: Pyrolysis is the only process that yields solid, liquid, and gaseous products, offering the greatest potential for economic and environmental benefits.
Ultimately, the right process is the one that most efficiently converts your input material into the specific output you desire.
Summary Table:
| Process | Oxygen Level | Primary Goal | Main Products |
|---|---|---|---|
| Combustion | Excess | Immediate Heat | Heat, CO₂, H₂O |
| Gasification | Restricted | Create Syngas Fuel | Syngas (H₂, CO) |
| Pyrolysis | None | Value-Added Products | Bio-char, Bio-oil, Syngas |
Ready to select the optimal thermal processing solution for your lab or project? The choice between combustion, gasification, and pyrolysis is critical for achieving your efficiency and sustainability goals. KINTEK specializes in lab equipment and consumables, providing the precise technology you need to convert materials effectively. Our experts can help you navigate the complexities of each process. Contact us today to discuss your specific application and discover the right equipment for your laboratory needs.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What is the application of vacuum brazing? Achieve Unmatched Joint Integrity for Critical Components
- What is the principle of vacuum furnace? Achieve Purity and Precision in Material Processing
- What materials are used in an electric arc furnace? A Guide to Scrap, Electrodes & Refractories
- What is the importance of vacuum ovens in electrode drying? Enhance Supercapacitor Performance with Precise Thermal Control
- What is the purpose of using a precision high-temperature aging furnace in hydrogen embrittlement research?
- What is physical Vapour deposition in crystal growth? Master Atomic-Level Thin Film Fabrication
- Why is a vacuum annealing furnace required for Inconel 713LC & 738? Ensure Peak Superalloy Performance
- What is the purpose of a sintering furnace? Create High-Performance Components Without Melting



















