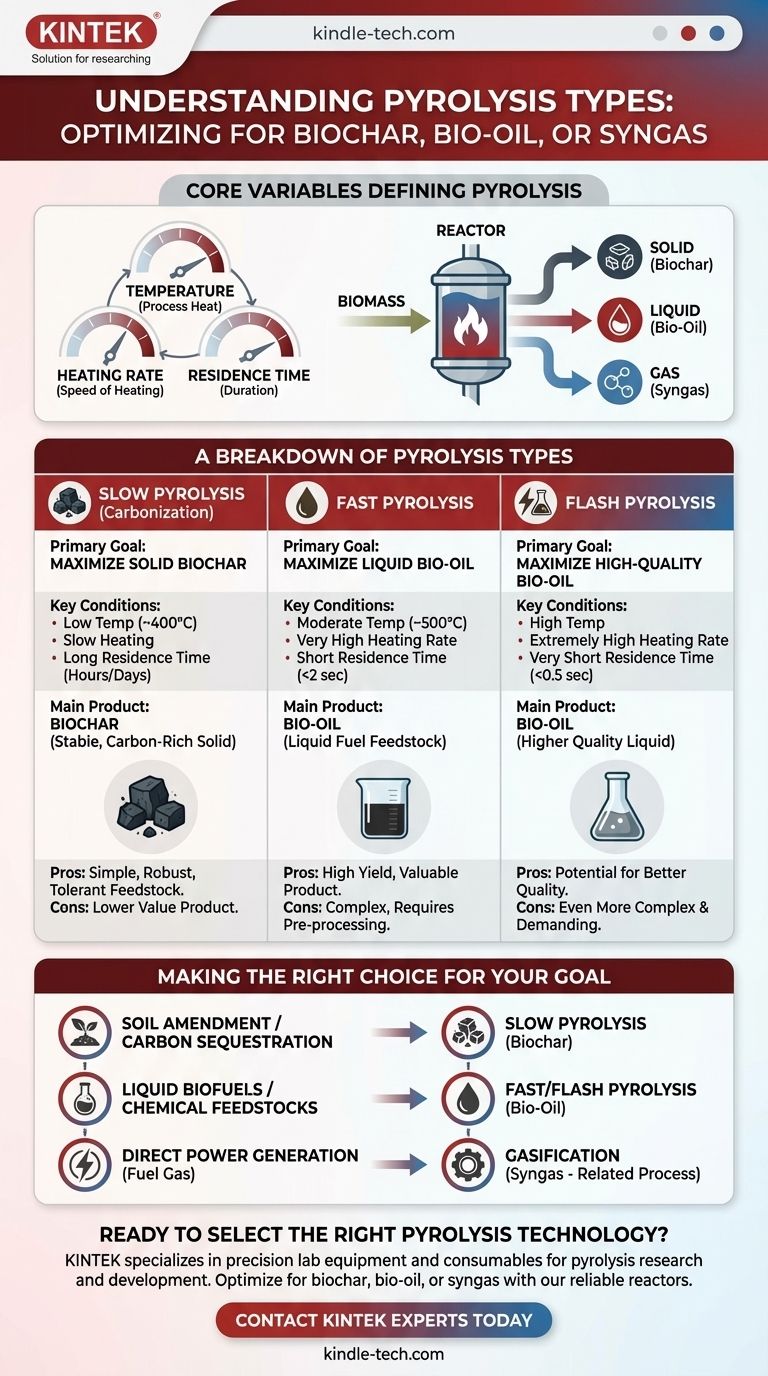
At its core, pyrolysis is a thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen. The key difference between its various types—slow, fast, and flash—lies in three critical parameters: temperature, heating rate, and residence time. These variables are deliberately controlled to favor the production of one of three primary products: a solid (biochar), a liquid (bio-oil), or a gas (syngas).
The fundamental distinction is a matter of process intent. Slow pyrolysis is engineered to maximize solid biochar, while fast and flash pyrolysis are optimized to produce the highest possible yield of liquid bio-oil.

The Core Variables That Define Pyrolysis
To understand the difference between pyrolysis types, you must first understand the three levers that control the outcome of the process. Manipulating these variables determines the final product distribution.
Heating Rate
This is the speed at which the feedstock is heated to the target temperature. A very high heating rate is the defining characteristic of fast and flash pyrolysis, favoring the rapid breakdown of organic matter into vapors that can be condensed into bio-oil.
Temperature
The process temperature determines which chemical bonds break. Lower temperatures (350-500°C) tend to favor solid char formation, while very high temperatures (>700°C) favor the cracking of all components into permanent gases.
Residence Time
This refers to the duration the feedstock spends at the reaction temperature. Long residence times, often hours, allow for secondary reactions that increase the formation of stable, solid biochar. In contrast, short residence times of just a few seconds are crucial for capturing valuable vapors before they break down further.
A Breakdown of Pyrolysis Types
The specific combination of these variables gives rise to distinct pyrolysis methods, each with a different primary output and application.
Slow Pyrolysis (Carbonization)
This is the oldest and most straightforward method, historically used to produce charcoal from wood. It uses low temperatures (around 400°C) and a very slow heating rate over a long residence time of several hours or even days.
These conditions allow the organic material to slowly decompose and rearrange its carbon structure, maximizing the output of biochar, a stable, carbon-rich solid.
Fast Pyrolysis
This process is engineered to maximize the production of liquid bio-oil. It involves moderate temperatures (around 500°C), a very high heating rate, and an extremely short residence time of less than two seconds.
The goal is to rapidly break down the biomass into vapors and then quickly cool (quench) them to prevent further reactions. This process converts the bulk of the feedstock into a liquid bio-oil, with smaller amounts of biochar and syngas as byproducts.
Flash Pyrolysis
Flash pyrolysis is an even more extreme and rapid variant of fast pyrolysis. It operates at slightly higher temperatures with extremely high heating rates and a vapor residence time of less than half a second.
The primary advantage, as noted in process research, is the potential for higher yields of bio-oil that may serve as a better, less-degraded feedstock for upgrading into conventional fuels and chemicals.
Understanding the Trade-offs
Choosing a pyrolysis method involves balancing process complexity against the desired product. Each approach comes with distinct operational requirements and challenges.
Product Value vs. Process Complexity
Slow pyrolysis is generally a simpler, more robust, and lower-cost process. However, its primary product, biochar, has a lower market value per unit of mass compared to liquid fuels.
Fast and flash pyrolysis systems are more complex and require precise control over temperature and residence time. This complexity is justified by the production of a higher-value, energy-dense liquid fuel.
Feedstock Requirements
The need for rapid heat transfer means fast and flash pyrolysis require the feedstock to be dried and finely ground (typically less than 2mm). This pre-processing adds to the operational cost.
Slow pyrolysis is far more tolerant of larger particle sizes and higher moisture content, reducing the need for extensive feedstock preparation.
Energy Balance
A key advantage of all pyrolysis systems is their potential for energy self-sufficiency. The non-condensable syngas produced during the process is a valuable fuel that can be combusted to provide the heat needed to run the reactor, significantly improving the overall energy balance.
Making the Right Choice for Your Goal
The optimal pyrolysis method is entirely dependent on your end goal. The process is not a one-size-fits-all solution; it is a targeted tool for converting biomass into a specific desired product.
- If your primary focus is soil amendment or carbon sequestration: Slow pyrolysis is the correct choice, as it is specifically designed to maximize the yield of stable, solid biochar.
- If your primary focus is producing liquid biofuels or chemical feedstocks: Fast or flash pyrolysis is the only viable path, as these processes are optimized to generate the highest possible yield of bio-oil.
- If your primary focus is generating fuel gas for direct power generation: While pyrolysis produces syngas, gasification (a related process at higher temperatures with a small amount of oxygen) is a more direct and efficient method.
Ultimately, understanding the interplay between heating rate, temperature, and residence time empowers you to select the right conversion technology for your specific objective.
Summary Table:
| Pyrolysis Type | Primary Goal | Key Conditions | Main Product |
|---|---|---|---|
| Slow Pyrolysis | Maximize solid yield | Low temp (~400°C), slow heating, long residence (hours) | Biochar |
| Fast Pyrolysis | Maximize liquid yield | Moderate temp (~500°C), very fast heating, short residence (<2 sec) | Bio-Oil |
| Flash Pyrolysis | Maximize high-quality liquid yield | High temp, extremely fast heating, very short residence (<0.5 sec) | Bio-Oil |
Ready to select the right pyrolysis technology for your biomass conversion goals? KINTEK specializes in precision lab equipment and consumables for pyrolysis research and development. Whether you're optimizing for biochar, bio-oil, or syngas production, our reactors and analytical tools provide the control and reliability you need. Contact our experts today to discuss how we can support your laboratory's specific pyrolysis applications and help you achieve superior process outcomes.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What role does a high-pressure reactor play in the hydrodeoxygenation (HDO) of bio-oil? Drive Deep Fuel Upgrading
- Why use high-pressure reactors for food waste pretreatment? Boost Hydrogen Production Efficiency Today!
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery
- What are the technical characteristics of PTFE (Teflon) lined hydrothermal reactors? Comparing α-ZrP Synthesis Methods
- What is the function of a PTFE-lined hydrothermal autoclave in cys-CDs synthesis? Achieve High-Purity Carbon Dots


















