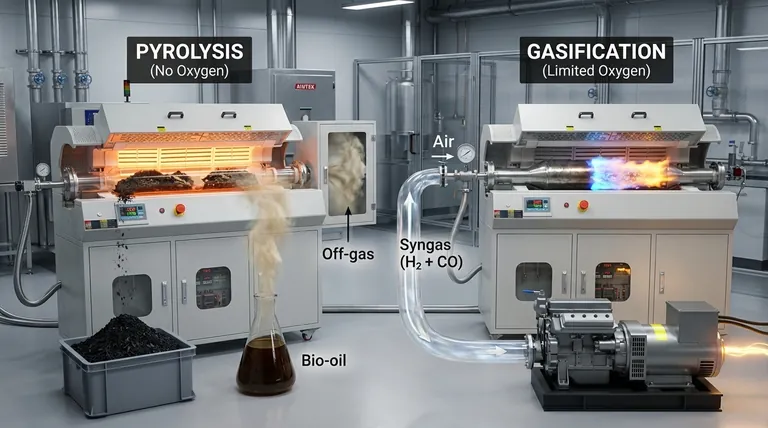
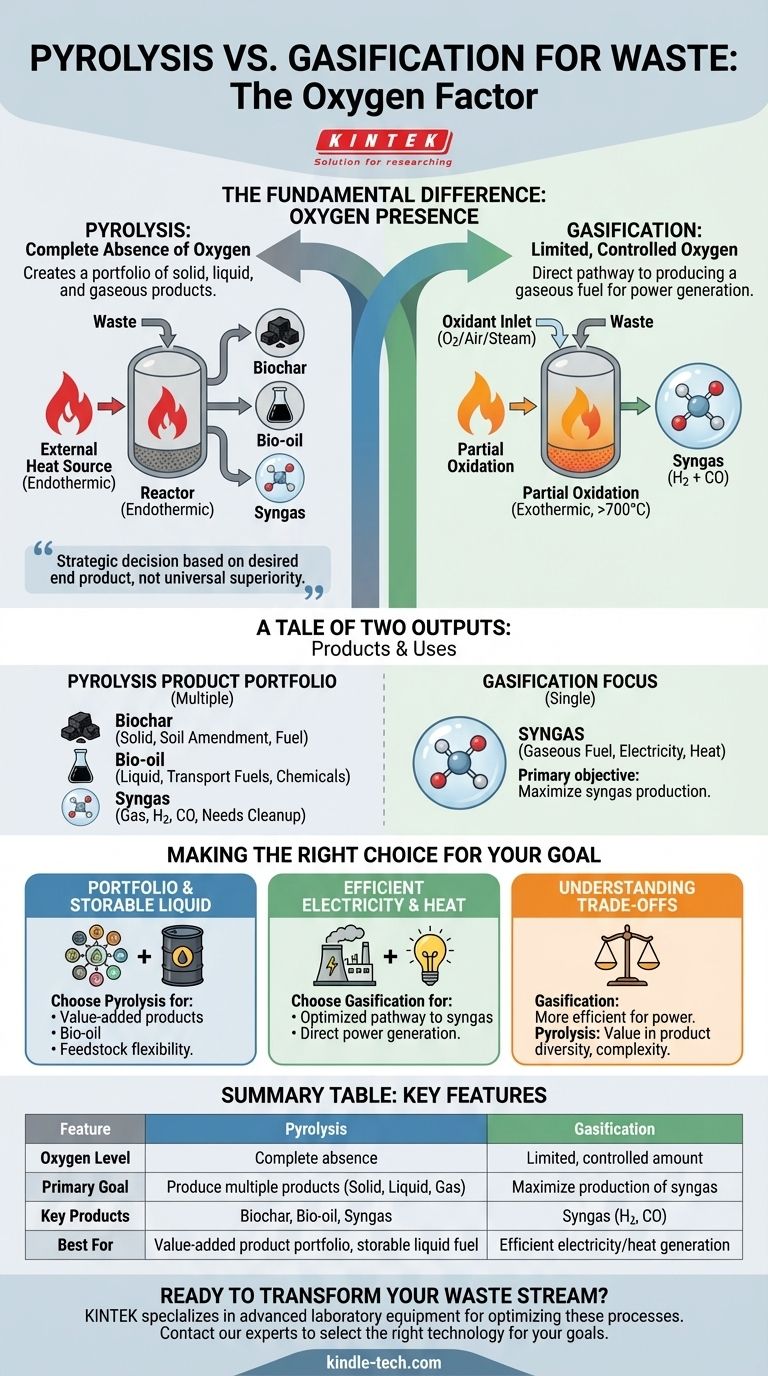
The fundamental difference between pyrolysis and gasification lies in the presence of oxygen. Pyrolysis is the thermal decomposition of waste in the complete absence of oxygen, breaking it down into solid char, liquid oil, and gas. In contrast, gasification uses a controlled, limited amount of oxygen to intentionally convert waste almost entirely into a combustible gas mixture known as syngas.
The choice between pyrolysis and gasification is not a matter of which process is universally "better," but a strategic decision based on the desired end product. Pyrolysis creates a portfolio of solid, liquid, and gaseous products, while gasification is a more direct pathway to producing a gaseous fuel for power generation.
The Defining Factor: The Role of Oxygen
The presence or absence of an oxidant, typically oxygen, is the core chemical distinction that dictates everything else about these two processes.
Pyrolysis: Thermal Decomposition Without Air
Pyrolysis is a strictly thermal process that heats organic materials in an oxygen-free environment. Because no oxidation occurs, it is primarily an endothermic process, meaning it requires a consistent external heat source to drive the reaction.
This absence of combustion ensures that the resulting products—solid char, liquid bio-oil, and gas—retain a high energy content derived from the original feedstock.
Gasification: Partial Oxidation with Limited Air
Gasification introduces a controlled amount of oxygen, steam, or air into the reactor. This is not enough for full combustion but is sufficient to trigger partial oxidation of the waste.
This limited combustion generates its own heat, making the process partially exothermic and typically operating at higher temperatures (above 700°C). The goal is not to burn the material, but to use the reaction to convert the solid feedstock into a gas.
A Tale of Two Outputs: Products and Their Uses
The difference in process chemistry leads to fundamentally different product streams, each with unique applications.
The Pyrolysis Product Portfolio: Solids, Liquids, and Gases
Pyrolysis breaks down waste into three distinct and valuable product streams:
- Biochar (Solid): A carbon-rich, charcoal-like substance used for soil amendment, filtration, or as a solid fuel.
- Bio-oil (Liquid): A dense, acidic liquid that can be upgraded into transportation fuels or used to produce specialty chemicals.
- Syngas (Gas): A mixture of hydrogen and carbon monoxide, but often containing other hydrocarbon compounds that may require an additional step, like catalytic reforming, for cleanup.
The Gasification Focus: Producing Syngas
The primary objective of gasification is to maximize the production of one specific output: synthesis gas (syngas).
This syngas is composed mainly of hydrogen (H2) and carbon monoxide (CO). Its primary application is as a clean, combustible fuel that can be used directly in gas engines or turbines to generate electricity and heat.
Understanding the Trade-offs
Choosing a technology requires acknowledging its inherent operational differences and limitations.
The Question of Energy Efficiency
For the direct production of electricity and heat, gasification is generally considered more efficient. The process is a more streamlined conversion of a solid feedstock into a ready-to-use fuel gas.
Pyrolysis is less efficient for direct power generation because the energy from the original waste is distributed across three different products (solid, liquid, and gas), each requiring potential further processing.
Process Complexity and Downstream Requirements
The value of pyrolysis comes from its product diversity, but this can also introduce complexity. Upgrading bio-oil into a usable fuel is a significant refining challenge, and the raw pyrolysis gas often requires cleanup before use.
Gasification, while conceptually simpler in its output, demands precise control over temperature and oxygen levels to ensure the consistent production of high-quality syngas and avoid undesirable byproducts.
Making the Right Choice for Your Goal
The optimal technology is defined by your strategic objective for the waste feedstock.
- If your primary focus is creating a portfolio of value-added products: Pyrolysis is the clear choice, offering distinct solid (biochar), liquid (bio-oil), and gaseous outputs.
- If your primary focus is generating electricity or heat as efficiently as possible: Gasification provides a more direct and optimized pathway to producing a combustible syngas fuel.
- If your primary focus is feedstock flexibility and producing a storable liquid fuel: Pyrolysis offers a unique advantage by converting waste into bio-oil, which can be stored and transported more easily than gas.
Ultimately, your choice depends not on the technology itself, but on the specific value you aim to extract from your waste stream.
Summary Table:
| Feature | Pyrolysis | Gasification |
|---|---|---|
| Oxygen Level | Complete absence | Limited, controlled amount |
| Primary Goal | Produce multiple products (solid, liquid, gas) | Maximize production of syngas |
| Key Products | Biochar, Bio-oil, Syngas | Syngas (Hydrogen, Carbon Monoxide) |
| Best For | Value-added product portfolio, storable liquid fuel | Efficient electricity/heat generation |
Ready to transform your waste stream into valuable energy or products? The choice between pyrolysis and gasification is critical for maximizing your return. KINTEK specializes in advanced laboratory equipment and consumables for researching and optimizing these thermal conversion processes. Our experts can help you select the right technology for your specific feedstock and output goals. Contact our team today to discuss your project and discover how KINTEK's solutions can power your innovation.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- For what purpose is an ultra-low temperature freezer used prior to oxide experiments? Ensure Atomic-Level Sample Purity
- What is the unit of measurement for melting point? Celsius, Kelvin, or Fahrenheit?
- What is sputtering yield? Master the Key to Efficient Thin Film Deposition
- What are three biomass materials that are pelletized? Wood, Agricultural Waste & Energy Crops
- What are the advantages of powder metallurgy parts? Achieve Cost-Effective, High-Performance Components
- What are the precautions to be taken while sampling? Ensure Data Accuracy and Minimize Bias
- How do ULT freezers achieve ultralow temperatures? A Deep Dive into Cascade Refrigeration
- Is graphite used in aerospace? Discover the Power of Carbon Fiber Composites



















