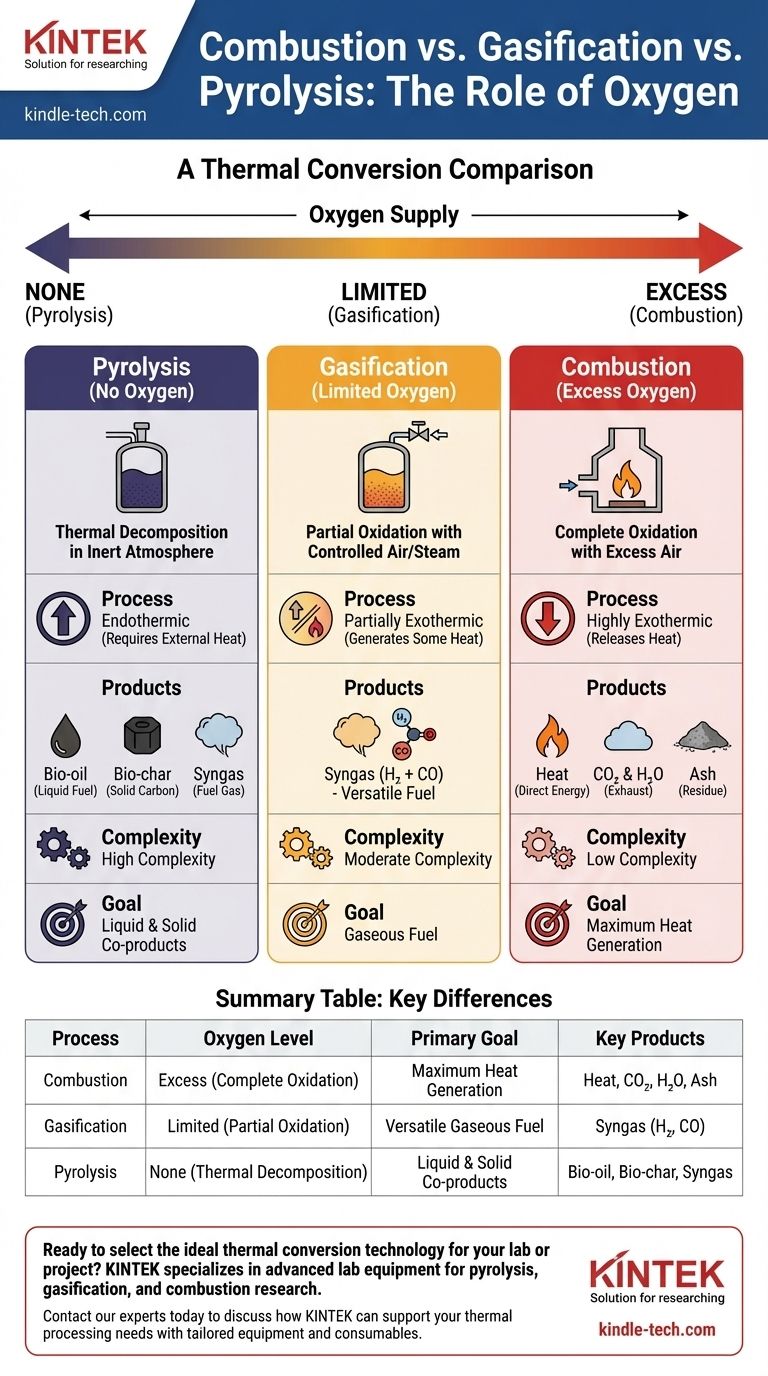
At its core, the difference between combustion, gasification, and pyrolysis is the amount of oxygen supplied during the process. Combustion involves complete oxidation in an oxygen-rich environment to release heat. Gasification uses a limited amount of oxygen to convert material into a combustible gas, and pyrolysis uses no oxygen at all, thermally decomposing material into a mix of liquid, solid, and gaseous products.
The choice between these thermal conversion technologies is not about finding the "best" one, but about defining your desired output. Your goal determines the process: direct heat (combustion), a versatile gaseous fuel (gasification), or a mix of valuable liquid and solid co-products (pyrolysis).

The Defining Factor: Oxygen's Role
The presence—or absence—of oxygen fundamentally changes the chemical reactions and the resulting products. Each process exists on a spectrum of oxidation.
Combustion: Complete Oxidation for Maximum Heat
Combustion is the process of rapid, complete oxidation. It requires a sufficient supply of an oxidant, typically air, to fully burn the organic material.
The primary goal of combustion is to release the maximum amount of the material's stored chemical energy as heat. The main byproducts are carbon dioxide (CO₂) and water (H₂O).
Gasification: Partial Oxidation for Gaseous Fuel
Gasification operates in an oxygen-starved environment, using only a controlled or limited amount of oxygen, sometimes with steam. This prevents complete combustion.
Instead of releasing all the energy as heat, this process converts the solid organic material into a combustible fuel gas known as synthesis gas, or syngas. This gas is primarily a mixture of hydrogen (H₂) and carbon monoxide (CO).
Pyrolysis: Thermal Decomposition Without Oxygen
Pyrolysis occurs in the complete absence of oxygen. The material is not burned; it is chemically decomposed by heat alone in an inert atmosphere.
Because no oxidation occurs, pyrolysis is an endothermic process, meaning it requires an external heat source to drive the reaction. The goal is to break down complex organic materials into simpler, valuable chemical components.
A Comparison of Outputs: What Each Process Creates
The different chemical environments lead to dramatically different and distinct product slates.
The Products of Combustion
Combustion is the least complex process in terms of outputs. It is designed to produce one primary product: usable heat. The other outputs are exhaust gases (flue gas), primarily CO₂ and water, along with ash.
The Products of Gasification
Gasification's primary output is syngas. This is a versatile intermediate product that can be burned in a gas engine or turbine to generate electricity or serve as a chemical building block to produce liquid fuels and other chemicals.
The Products of Pyrolysis
Pyrolysis produces three distinct types of products, all of which retain a high energy content:
- Bio-oil (or Pyrolysis Oil): A dark, viscous liquid fuel that can be refined or used in certain engines and boilers.
- Bio-char: A stable, carbon-rich solid similar to charcoal that can be used as a fuel or as a valuable soil amendment.
- Syngas: A mixture of gases, including flammable hydrocarbons, carbon monoxide, and hydrogen. This gas often requires further processing (reforming) to be used as a clean fuel.
Understanding the Trade-offs
Choosing a technology requires acknowledging the inherent trade-offs in process complexity, energy balance, and product versatility.
Process Complexity and Control
Combustion is the simplest and most mature of the three technologies. Gasification requires more sophisticated control over oxygen and temperature to optimize syngas quality.
Pyrolysis is the most sensitive process. It demands strict control to prevent any oxygen from entering the system and precise temperature management to influence the ratio of bio-oil, bio-char, and gas produced.
Energy Input vs. Output
Combustion is a highly exothermic process; it releases a large amount of energy as heat.
Gasification is partially exothermic, generating some of its own process heat. Pyrolysis, being endothermic, requires a constant and significant external energy input to maintain the reaction.
Product Versatility
Combustion offers the lowest versatility, producing only heat. Gasification is more flexible, as its syngas output can be used for power generation or as a chemical feedstock.
Pyrolysis offers the highest product versatility. The ability to create a liquid fuel (bio-oil), a solid product (bio-char), and a fuel gas from a single process makes it a powerful tool for biorefineries and waste-to-value applications.
Making the Right Choice for Your Goal
Your selection should be guided by the specific product you need to generate from your organic material.
- If your primary focus is direct, maximum heat generation: Combustion is the most direct, efficient, and simplest pathway.
- If your primary focus is creating a flexible, clean-burning gaseous fuel for power or chemical synthesis: Gasification is the ideal choice.
- If your primary focus is producing valuable liquid fuels, chemical precursors, or solid carbon products: Pyrolysis provides the unique capability to create these high-value materials.
By understanding the fundamental role of oxygen, you can select the precise thermal process to convert organic materials into the valuable products you need.
Summary Table:
| Process | Oxygen Level | Primary Goal | Key Products |
|---|---|---|---|
| Combustion | Excess (Complete Oxidation) | Maximum Heat Generation | Heat, CO₂, H₂O, Ash |
| Gasification | Limited (Partial Oxidation) | Versatile Gaseous Fuel | Syngas (H₂, CO) |
| Pyrolysis | None (Thermal Decomposition) | Liquid & Solid Co-products | Bio-oil, Bio-char, Syngas |
Ready to select the ideal thermal conversion technology for your lab or project? KINTEK specializes in advanced lab equipment for pyrolysis, gasification, and combustion research. Whether you're developing sustainable fuels, analyzing waste-to-value processes, or optimizing energy recovery, our solutions deliver precise temperature control, robust performance, and reliable data. Contact our experts today to discuss how KINTEK can support your thermal processing needs with tailored equipment and consumables.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the different types of pyrolysis machines? Choose the Right System for Your Output


















