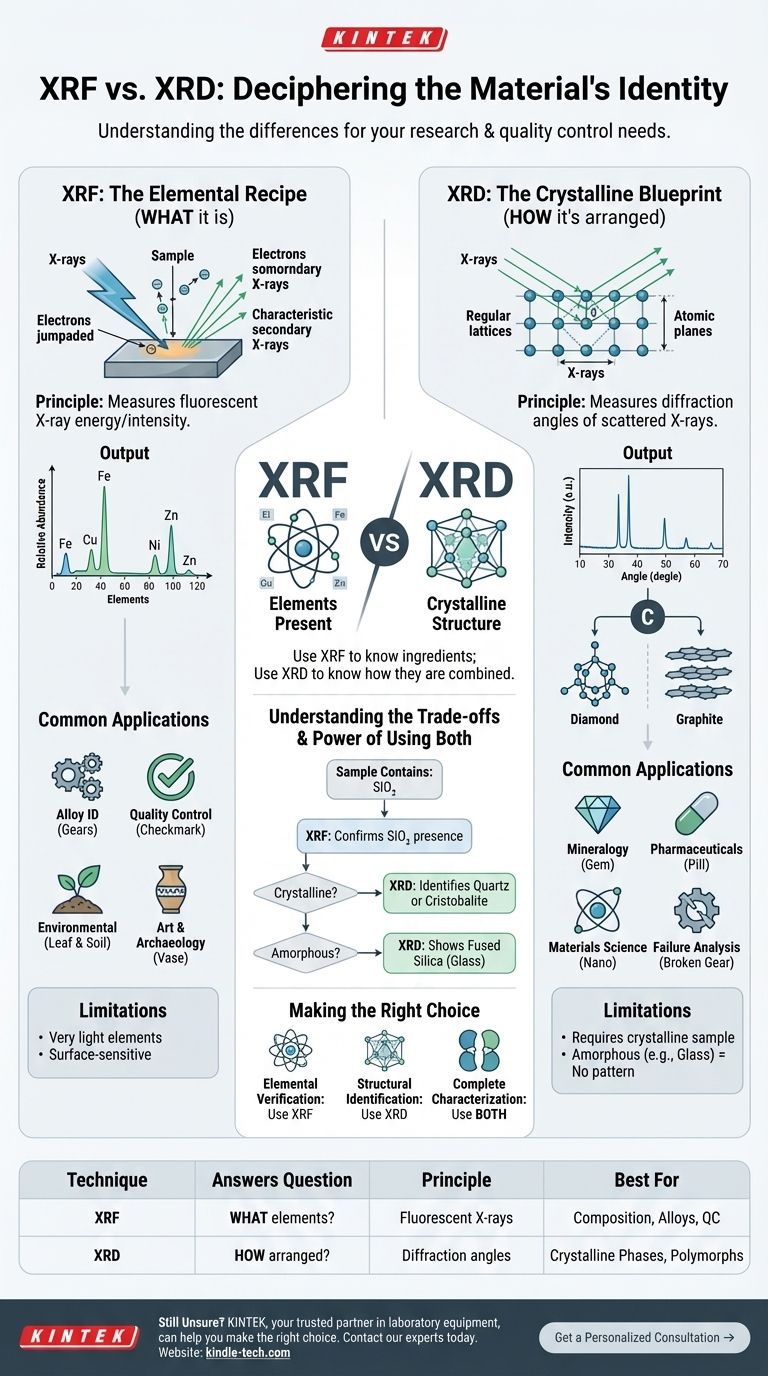
At their core, X-ray Fluorescence (XRF) and X-ray Diffraction (XRD) answer two fundamentally different questions about a material. XRF identifies the chemical elements present and their relative amounts, while XRD identifies the crystalline structure, or how those atoms are arranged into specific compounds or phases.
The simplest way to distinguish them is to think of XRF as identifying what a material is made of (its elemental ingredients), whereas XRD identifies how those ingredients are put together (its crystalline structure or phase).

What XRF Tells You: The Elemental Recipe
X-ray Fluorescence is a non-destructive technique used for elemental analysis. It operates by bombarding a sample with high-energy primary X-rays.
The Fundamental Principle
When the primary X-ray strikes an atom in your sample, it can knock an electron out of an inner orbital. This creates an unstable vacancy, which is immediately filled by an electron from a higher-energy outer orbital.
As this electron "falls" into the vacancy, it releases a secondary, or "fluorescent," X-ray. The energy of this fluorescent X-ray is unique to the element from which it originated, acting like an elemental fingerprint.
The Output: A List of Elements
The XRF detector measures the energies and intensities of all the fluorescent X-rays coming from the sample.
This produces a spectrum that tells you which elements are present (e.g., iron, copper, nickel, zinc) and, by measuring the intensity, their approximate concentration. It essentially provides an elemental parts list for your material.
Common Applications of XRF
- Alloy Identification: Quickly verifying the grade and composition of metals.
- Quality Control: Ensuring raw materials meet elemental specifications.
- Environmental Screening: Testing soil for heavy metal contamination like lead or arsenic.
- Art & Archaeology: Analyzing the elemental makeup of pigments or artifacts without damage.
What XRD Tells You: The Crystalline Blueprint
X-ray Diffraction is a technique used to determine the atomic and molecular structure of a crystalline material. It does not primarily identify elements.
The Fundamental Principle
XRD works by directing a beam of X-rays onto a sample and measuring the angles at which the beam is scattered or "diffracted." For this to occur, the material must be crystalline, meaning its atoms are arranged in a regular, repeating lattice.
This diffraction only happens at specific angles where the scattered X-rays interfere constructively, a phenomenon described by Bragg's Law. The angles are directly related to the spacing between the planes of atoms in the crystal lattice.
The Output: A Structural Fingerprint
The result of an XRD scan is a diffractogram, which plots X-ray intensity against the angle of diffraction. This pattern is a unique fingerprint for a specific crystalline structure.
For example, both diamond and graphite are pure carbon (XRF would just show "Carbon"). However, their XRD patterns are completely different because their atoms are arranged in vastly different crystal structures. XRD can distinguish between them, identifying one as "diamond" and the other as "graphite."
Common Applications of XRD
- Mineralogy: Identifying the specific minerals present in a rock sample.
- Pharmaceuticals: Distinguishing between polymorphs (different crystal forms of the same drug), which can have different bioavailabilities.
- Materials Science: Determining the crystal phases present in a synthesized material, ceramic, or polymer.
- Failure Analysis: Identifying corrosion products or unexpected phases in a failed component.
Understanding the Trade-offs
Neither technique is universally superior; their value depends entirely on the question you need to answer. Understanding their limitations is key to using them effectively.
XRF Limitations
XRF is very poor at detecting very light elements (typically those lighter than sodium, Na), such as carbon, oxygen, nitrogen, and lithium. It is also primarily a surface-sensitive technique, so the bulk composition may differ if the sample is not homogeneous.
XRD Limitations
The biggest limitation of XRD is that it requires a crystalline sample. Amorphous materials, like glass or many polymers, do not have the ordered atomic structure needed for diffraction and will not produce a distinct pattern. Furthermore, analyzing complex mixtures of multiple crystalline phases can be challenging.
The Power of Using Both
XRF and XRD are exceptionally powerful when used together. XRF can tell you that a sample contains Silicon and Oxygen. XRD can then tell you if that SiO₂ is present as crystalline quartz, cristobalite, or if it is amorphous fused silica (glass).
Making the Right Choice for Your Goal
To select the correct method, you must first define your analytical goal.
- If your primary focus is elemental verification: Use XRF to confirm the elemental composition of an alloy, check for restricted heavy metals, or quantify major elements.
- If your primary focus is structural identification: Use XRD to identify the specific mineral or compound, check for unwanted crystalline phases, or confirm the structure of a synthesized material.
- If your primary focus is complete characterization: Use both. Start with XRF to get the elemental makeup, then use XRD to understand how those elements are structurally combined.
Choosing the right tool begins with asking the right question about your material.
Summary Table:
| Technique | Answers the Question | Principle | Best For |
|---|---|---|---|
| XRF | What elements are present? | Measures fluorescent X-rays from the sample. | Elemental composition, alloy ID, quality control. |
| XRD | How are the atoms arranged? | Measures diffraction angles from a crystal lattice. | Identifying crystalline phases, minerals, polymorphs. |
Still Unsure Which Technique is Right for Your Analysis?
Choosing between XRF and XRD is critical for accurate results. KINTEK, your trusted partner in laboratory equipment, can help you make the right choice. We specialize in providing the precise lab equipment and consumables you need for your specific analytical challenges.
Contact our experts today to discuss your application and discover the ideal solution for your lab. Let KINTEK empower your research and quality control with the right tools.
Get a Personalized Consultation →
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
People Also Ask
- What is a laboratory heater? A Guide to Precision, Safety, and Choosing the Right Type
- What are the major components of biomass? Unlocking the Building Blocks of Renewable Energy
- Can plastic waste be converted into fuel? Discover the methods, benefits, and risks.
- What is the mechanical function of an overhead digital stirrer? Optimize Molybdenum Disk Dissolution Efficiency
- What is the sintering process of coating? Building Durable, Solid Layers from Powder
- Is pyrolysis oil harmful? Understanding the Risks of This Reactive Biofuel
- What are the defects in sintered parts? Avoid Warping, Cracking, and Porosity Issues
- What is hot forging used for? Manufacturing Critical, High-Strength Metal Components



















