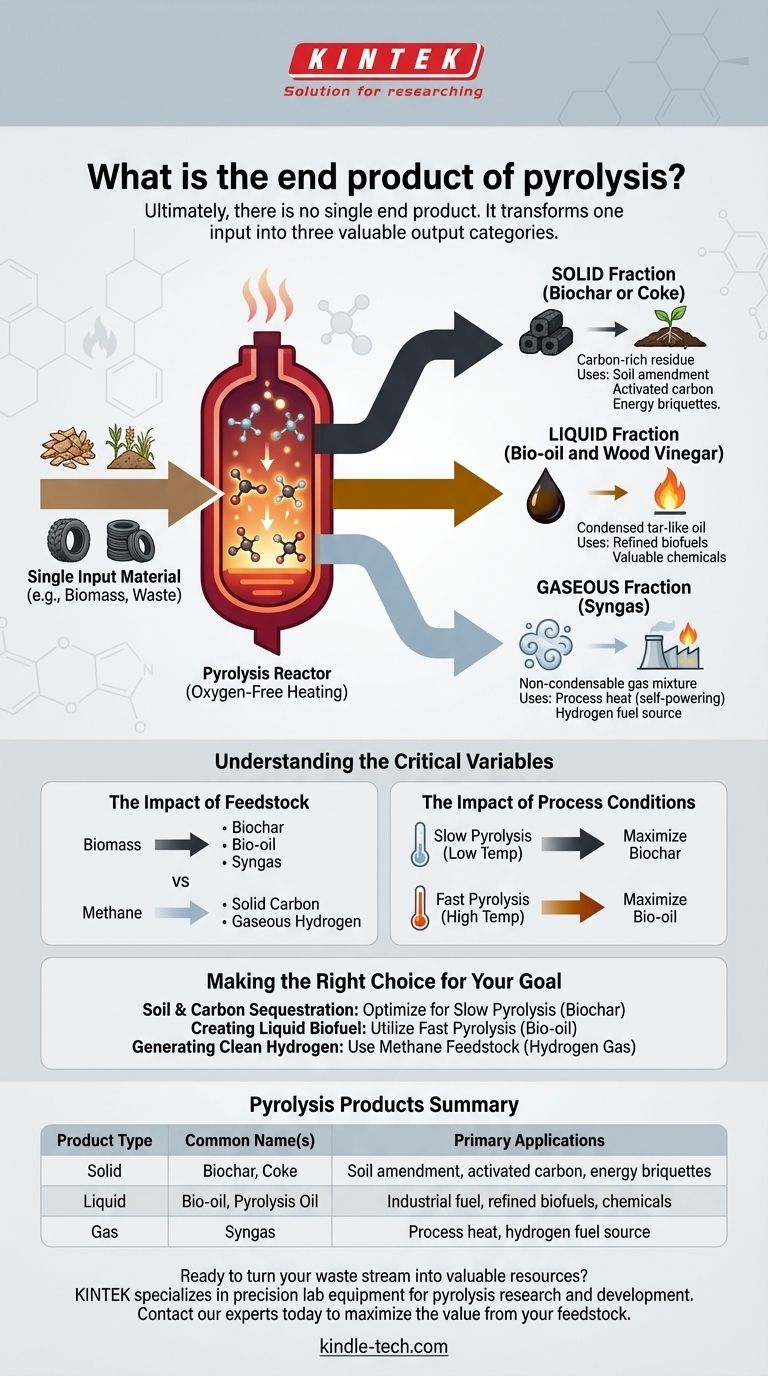
Ultimately, there is no single end product of pyrolysis. Instead, the process transforms a single input material into three distinct categories of valuable outputs: a solid, a liquid, and a gas. The exact composition and proportion of these products are determined by the initial material being processed and the specific conditions of the pyrolysis reaction, such as temperature and heating rate.
Pyrolysis is best understood not as a process with one outcome, but as a controlled thermal decomposition that fractures organic materials in the absence of oxygen, reliably yielding a carbon-rich solid (biochar), a complex liquid (bio-oil), and a combustible gas (syngas).

What Drives the Pyrolysis Outcome?
To understand the products, you must first understand the process. Pyrolysis is the act of heating organic material to a high temperature in an oxygen-free or low-oxygen environment.
The Core Principle: Heat Without Burning
Instead of combusting (burning), the heat breaks down the complex molecules of the input material into simpler, smaller molecules. Because there is no oxygen, these smaller molecules don't ignite but instead form new substances.
The Three Product Categories
The output of this thermal cracking process always falls into three states of matter.
- Solid: A carbon-rich solid residue.
- Liquid: A dense, tar-like oil condensed from the vapor.
- Gas: A non-condensable gas mixture.
A Closer Look at the Pyrolysis Products
Each of the three product streams has unique properties and applications, turning what was often considered waste into a valuable resource.
The Solid Fraction: Biochar or Coke
This solid product is the carbon skeleton of the original material. It is often called biochar when derived from biomass or coke from other materials like coal or waste tires.
Its primary uses include soil amendment in agriculture, production of energy briquettes, and manufacturing of activated carbon for filtration.
The Liquid Fraction: Bio-oil and Wood Vinegar
As the process unfolds, hot vapors are released. When these vapors are cooled and condensed, they form a liquid commonly known as pyrolysis oil or bio-oil.
This complex liquid can be used directly as an industrial fuel or further refined into higher-grade biofuels and valuable chemicals. When biomass is the feedstock, specific condensates like wood vinegar can also be collected.
The Gaseous Fraction: Syngas
The remaining vapors that do not condense into a liquid form a mixture of non-condensable gases, primarily syngas (synthesis gas).
This gas is rich in hydrogen, methane, and carbon monoxide. In most pyrolysis plants, this syngas is captured and used to provide the heat for the pyrolysis reactor itself, making the process highly energy-efficient.
Understanding the Critical Variables
The "end product" of pyrolysis is not fixed because it is a highly adaptable process. The operator can change the outcome by controlling two key factors.
The Impact of Feedstock
The material you put in fundamentally changes what you get out.
Pyrolyzing biomass like wood or agricultural waste will yield biochar, bio-oil, and syngas. In contrast, pyrolyzing a simple hydrocarbon like methane yields only two products: solid carbon and gaseous hydrogen.
The Impact of Process Conditions
Slight changes in temperature and heating speed can dramatically shift the product ratios.
Slow pyrolysis at lower temperatures maximizes the yield of the solid biochar. This is ideal for applications focused on carbon sequestration and soil health.
Fast pyrolysis at higher temperatures is optimized to break down the material into vapors, maximizing the yield of liquid bio-oil for fuel production.
Making the Right Choice for Your Goal
The versatility of pyrolysis means the "best" outcome is defined entirely by your objective. You can engineer the process to favor the specific product you need.
- If your primary focus is soil improvement and carbon sequestration: Optimize for slow, lower-temperature pyrolysis to maximize the solid biochar yield.
- If your primary focus is creating liquid biofuel: Utilize fast pyrolysis at moderate temperatures to maximize the condensable bio-oil fraction.
- If your primary focus is generating clean hydrogen fuel: Use a specific feedstock like methane to produce pure hydrogen gas and solid carbon.
By controlling the inputs and conditions, pyrolysis becomes a powerful tool for converting waste streams into precisely the resources you require.
Summary Table:
| Product Type | Common Name(s) | Primary Applications |
|---|---|---|
| Solid | Biochar, Coke | Soil amendment, activated carbon, energy briquettes |
| Liquid | Bio-oil, Pyrolysis Oil | Industrial fuel, refined biofuels, chemicals |
| Gas | Syngas | Process heat, hydrogen fuel source |
Ready to turn your waste stream into valuable resources? KINTEK specializes in precision lab equipment for pyrolysis research and development. Whether you're optimizing for biochar production, bio-oil yield, or syngas quality, our reactors and analytical tools provide the control you need. Contact our experts today to discuss how we can support your specific pyrolysis goals and help you maximize the value from your feedstock.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- Why must SCWG reactors maintain a specific heating rate? Protect Your High-Pressure Vessels from Thermal Stress
- What role does a high-pressure reactor play in the hydrodeoxygenation (HDO) of bio-oil? Drive Deep Fuel Upgrading
- Why are high-strength alloy tube reactors critical for HHIP? Ensuring Safety and Purity in High-Pressure Environments
- What are the technical characteristics of PTFE (Teflon) lined hydrothermal reactors? Comparing α-ZrP Synthesis Methods
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery

















