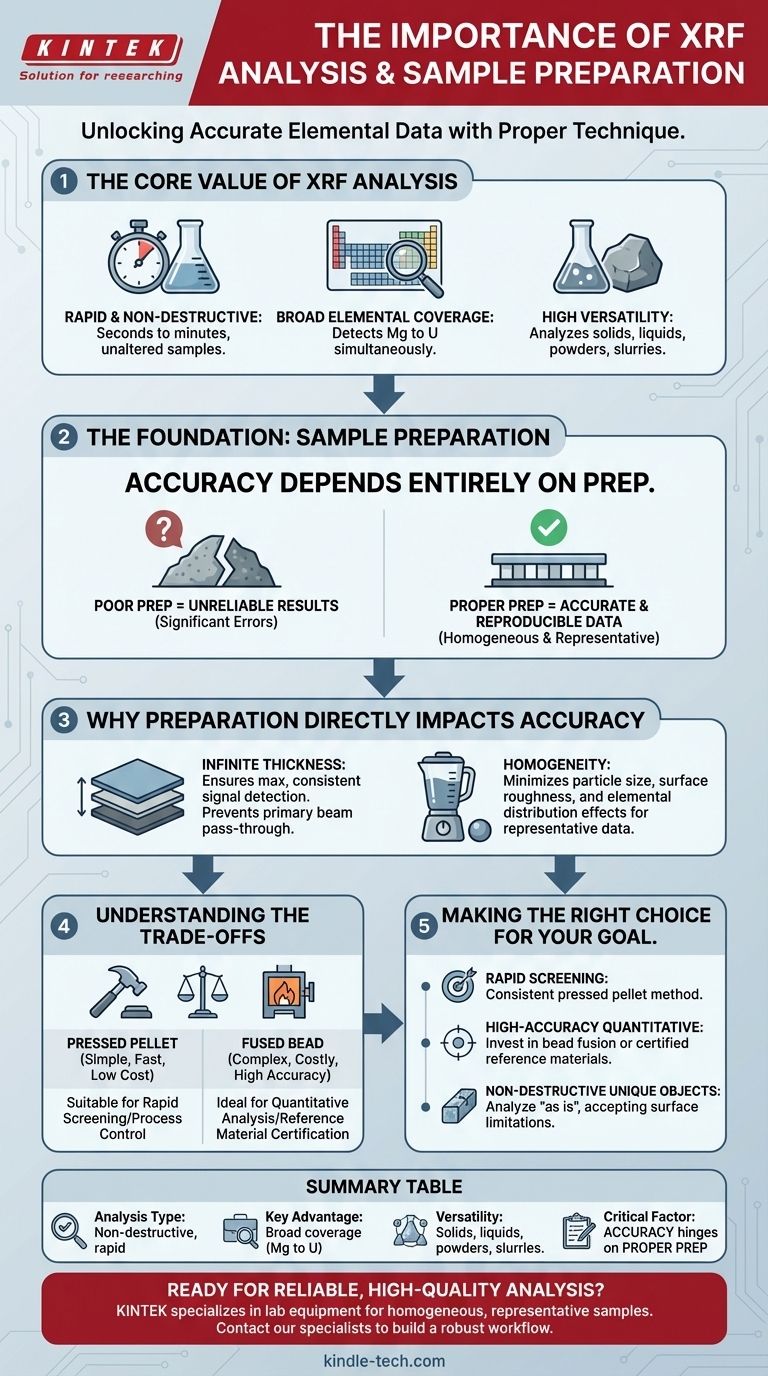
At its core, X-ray Fluorescence (XRF) analysis is a powerful and widely used technique for determining the elemental composition of a material. Its importance lies in its ability to provide rapid, non-destructive, and accurate chemical data for a vast range of sample types, from soils and metals to liquids and plastics. This makes it an indispensable tool for quality control, research, and regulatory compliance across dozens of industries.
The true value of XRF is its ability to deliver fast, reliable elemental analysis. However, the reliability of your results is not guaranteed by the instrument alone; it is almost entirely dependent on proper and consistent sample preparation.

The Core Value of XRF Analysis
To understand why meticulous preparation is so critical, we must first appreciate what makes XRF a go-to analytical method. Its importance is built on several key advantages.
Rapid and Non-Destructive Analysis
XRF is exceptionally fast, with measurements often taking seconds to minutes. Crucially, it is also non-destructive, meaning the sample is typically unaltered by the analysis. This is vital when analyzing valuable, rare, or irreplaceable materials.
Broad Elemental Coverage
A single XRF scan can identify and quantify a wide array of elements, from magnesium (Mg) to uranium (U), simultaneously. This provides a comprehensive chemical snapshot of the sample without requiring different procedures for different elements.
High Versatility
The technique can be applied to solids, liquids, powders, and slurries. This flexibility allows it to be deployed in diverse settings, from a laboratory analyzing prepared samples to a handheld device screening scrap metal in a salvage yard.
The Foundation of Accurate Results: Sample Preparation
While the XRF instrument performs the measurement, the quality of that measurement is determined long before the sample is placed inside. The references are unanimous: sample preparation is the most important step for achieving accurate and reproducible results. An unprepared or poorly prepared sample can render the results from a multi-million dollar instrument completely useless.
Why Preparation Directly Impacts Accuracy
The goal of sample preparation is to present the instrument with a sample that is homogeneous and representative of the bulk material. Incorrect preparation introduces significant errors.
Factors like particle size, surface roughness, and elemental distribution within the sample can dramatically alter the X-ray signal. Proper preparation minimizes these effects, ensuring the measurement reflects the sample's true composition.
The Principle of "Infinite Thickness"
For accurate results, the sample must be "infinitely thick." This does not mean it needs to be physically enormous. It means the sample must be thick enough that the primary X-ray beam cannot pass through it.
If a sample is too thin, the X-ray signal will be weaker and non-representative, as it doesn't capture the full interaction volume. Ensuring infinite thickness guarantees that the detected signal is maximized and consistent from one sample to the next.
Homogeneity: The Key to Representative Data
XRF analyzes a relatively small spot on the sample's surface. If that spot is not chemically identical to the rest of the material, the result will be incorrect.
Grinding powders or fusing a sample into a glass bead (a fused bead) are common methods to eliminate this variability and create a perfectly homogeneous sample for the instrument to analyze.
Understanding the Trade-offs
Choosing a sample preparation method involves a deliberate balance between the desired level of accuracy, the time and effort you can invest, and the cost.
Effort vs. Accuracy
A simple pressed powder pellet is fast and inexpensive but may suffer from particle size effects. Creating a fused bead is more complex and costly but eliminates most of these errors, yielding far more accurate results.
The choice depends entirely on your objective. Quick quality control might only require a pressed pellet, while certifying a reference material demands the precision of a fused bead.
Choosing the Right Method and Accessories
Your preparation choice also involves practical considerations. For loose powders or liquids, a thin film is used to contain the sample, and the material of this film must be chosen carefully to avoid contaminating the signal of the elements you want to measure.
When pressing powders, binders are often used to create a durable pellet that won't break. The binder itself must be free of the elements of interest and used in precise, repeatable quantities.
Making the Right Choice for Your Goal
The "best" sample preparation method is the one that meets the data quality objectives for your specific application.
- If your primary focus is rapid screening or process control: A consistent method for preparing pressed powder pellets is often sufficient.
- If your primary focus is high-accuracy quantitative analysis: You must invest in techniques like bead fusion or use certified reference materials to correct for matrix effects.
- If you are analyzing unique solid objects (e.g., artifacts, metal parts): Your goal is non-destructive analysis, so you will likely analyze the object "as is," accepting that surface condition may limit absolute accuracy.
Mastering sample preparation is how you unlock the full power and importance of XRF analysis.
Summary Table:
| Key Aspect | Importance of XRF Analysis |
|---|---|
| Analysis Type | Non-destructive, rapid elemental analysis |
| Key Advantage | Broad elemental coverage (Mg to U) |
| Versatility | Analyzes solids, liquids, powders, and slurries |
| Critical Factor | Accuracy depends entirely on proper sample preparation |
Ready to achieve reliable, high-quality elemental analysis in your lab?
The accuracy of your XRF results hinges on consistent and proper sample preparation. KINTEK specializes in the lab equipment and consumables—including presses, fusion furnaces, and high-purity binders—that are essential for creating homogeneous, representative samples. Whether your goal is rapid screening or high-accuracy quantitative analysis, our expertise ensures your XRF instrument delivers its full potential.
Contact us today to discuss your specific application and let our specialists help you build a robust sample preparation workflow. Get in touch via our contact form to get started.
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Three-dimensional electromagnetic sieving instrument
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
People Also Ask
- How do high-precision sieving systems benefit zeolite preparation? Maximize Adsorption for Wastewater Treatment
- What is the significance of using a fine sieving system for catalyst particles? Optimize Size for Maximum Reactivity
- Why is powder classification using standard sieves essential for SHS reactions? Unlock Superior Nitriding Results
- Why is a laboratory sieving system required for bentonite in coatings? Ensure Flawless Surface Performance
- What function does a sieving system perform during HPS powder pretreatment? Ensure Uniform Particle Size Distribution

















