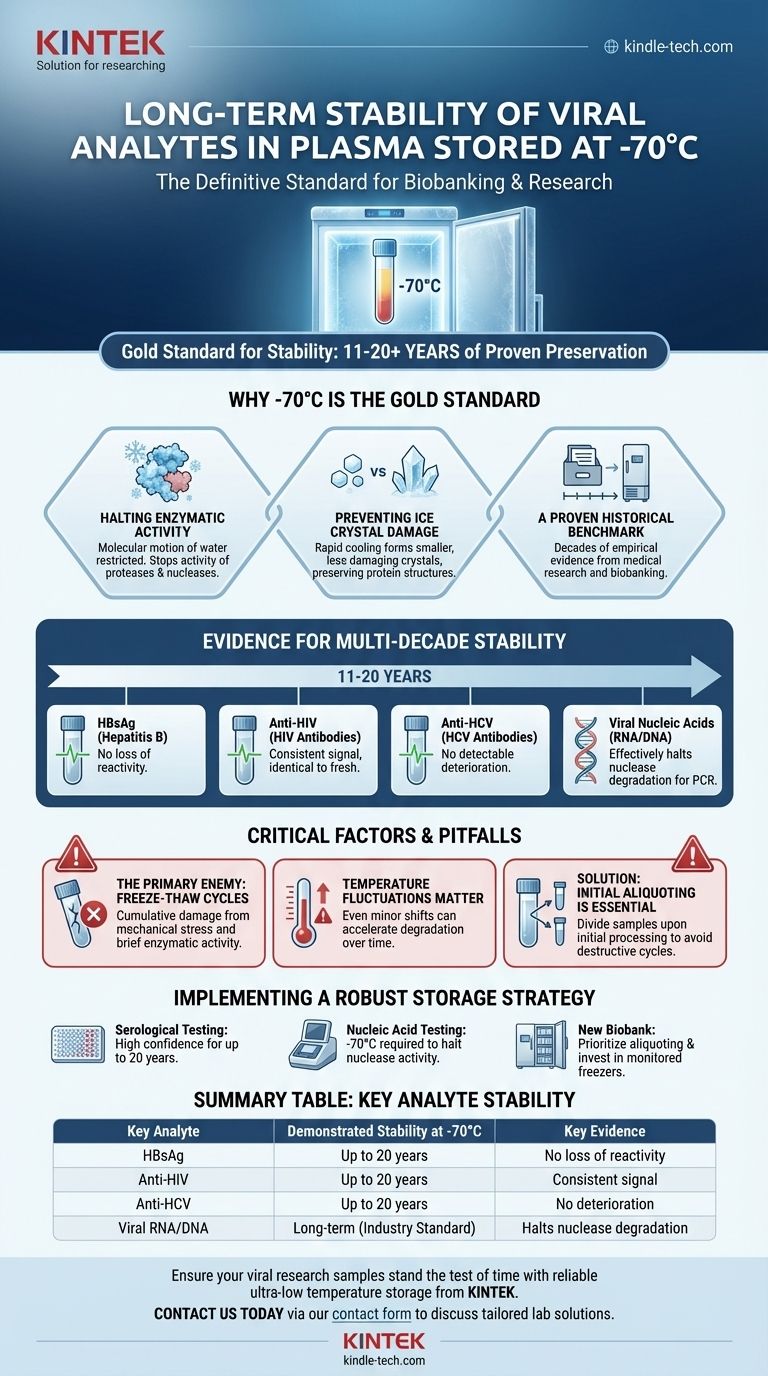
Based on extensive long-term studies, viral analytes in plasma demonstrate exceptional stability when stored at or below -70°C. Specific research tracking plasma samples for 11 to 20 years found no detectable deterioration or loss of reactivity for critical viral markers, including Hepatitis B surface antigen (HBsAg), antibodies to HIV (anti-HIV), and antibodies to HCV (anti-HCV). This makes -70°C the definitive standard for long-term viral biobanking.
The core principle is that at ultra-low temperatures, the biological and chemical processes that degrade samples are effectively frozen in time. This allows for the preservation of viral proteins, antibodies, and nucleic acids for decades, provided the storage conditions remain consistent.

Why -70°C is the Gold Standard for Stability
The choice of -70°C is not arbitrary; it is based on fundamental biophysical principles that halt sample degradation. This temperature serves as a critical threshold for preserving the molecular integrity of biological specimens.
Halting Enzymatic Activity
At temperatures around -70°C, the molecular motion of water becomes so restricted that it is essentially unavailable for chemical reactions. This state, known as the "glass transition" phase, effectively stops the activity of enzymes like proteases and nucleases that would otherwise break down viral proteins and genetic material.
Preventing Ice Crystal Damage
While freezing at any temperature creates ice crystals, the rapid cooling to ultra-low temperatures results in the formation of smaller, less-damaging crystals compared to storage at -20°C. This better preserves the delicate structures of proteins and antibodies.
A Proven Historical Benchmark
For decades, ultra-low temperature freezers set to -65°C or -70°C have been the cornerstone of medical research and biobanking. This long history provides a massive body of empirical evidence confirming the long-term stability of a wide range of biological analytes, including those from viruses.
Evidence for Multi-Decade Stability
The reliability of storage at -70°C is not just theoretical. It is supported by direct, long-term observational studies on invaluable patient samples.
Stability of Viral Proteins and Antibodies
The most direct evidence comes from studies on serological markers. In multiple reports, plasma samples stored consistently at -70°C for periods ranging from 11 to 20 years showed no loss in signal for HBsAg, anti-HIV, and anti-HCV. The samples behaved identically to freshly collected ones.
Stability of Viral Nucleic Acids
This same principle of halting enzymatic degradation applies to viral RNA and DNA. Ultra-low temperature storage is the standard for preserving viral genetic material for applications like PCR-based viral load testing, genotyping, and sequencing. It effectively deactivates the RNases and DNases present in plasma that would quickly destroy nucleic acids at warmer temperatures.
Understanding the Critical Factors and Pitfalls
Achieving multi-decade stability is not only about the temperature itself but also about the integrity of the storage process. Several factors can compromise the long-term viability of a sample.
The Primary Enemy: Freeze-Thaw Cycles
The single greatest threat to sample integrity is not the duration of storage, but the number of times it is thawed and refrozen. Each freeze-thaw cycle subjects proteins to mechanical stress from ice crystal formation and allows for brief periods of enzymatic activity, leading to cumulative damage and a potential loss of analyte reactivity.
Temperature Fluctuations Matter
Samples are most vulnerable during temperature fluctuations. A reliable ultra-low freezer with robust temperature monitoring, alarms, and a backup power source is essential. Even minor upward shifts in temperature, if sustained, can accelerate degradation over a period of years.
Initial Aliquoting is Essential
To avoid destructive freeze-thaw cycles, best practice dictates that samples should be divided into smaller volumes, or aliquots, upon initial processing. This allows researchers to thaw only the small amount needed for a specific experiment, leaving the master stock untouched and perfectly preserved.
Implementing a Robust Storage Strategy
Your approach to storage should be dictated by your long-term scientific or diagnostic goals. Proper planning ensures your samples remain a valuable resource for years to come.
- If your primary focus is serological testing (antibodies/antigens): You can have high confidence in results from samples stored consistently at -70°C for up to 20 years, as proven in long-term studies.
- If your primary focus is nucleic acid testing (e.g., viral load): -70°C is the required standard for long-term preservation, as it is the most effective way to halt nuclease activity that degrades viral RNA and DNA.
- If you are establishing a new biobank: Prioritize aliquoting all samples upon initial collection and invest in a high-quality, monitored ultra-low freezer to eliminate the risks of temperature fluctuations and freeze-thaw cycles.
Ultimately, consistent storage at -70°C transforms plasma from a perishable specimen into a stable, long-term asset for research and diagnostics.
Summary Table:
| Key Analyte | Demonstrated Stability at -70°C | Key Evidence |
|---|---|---|
| Hepatitis B surface antigen (HBsAg) | Up to 20 years | No loss of reactivity in long-term studies |
| Antibodies to HIV (anti-HIV) | Up to 20 years | Consistent signal identical to fresh samples |
| Antibodies to HCV (anti-HCV) | Up to 20 years | No detectable deterioration over decades |
| Viral Nucleic Acids (RNA/DNA) | Long-term (industry standard) | Effectively halts nuclease degradation |
Ensure your viral research samples stand the test of time with reliable ultra-low temperature storage from KINTEK.
As a specialist in laboratory equipment and consumables, KINTEK provides the robust ultra-low freezers and storage solutions you need to preserve the integrity of your plasma samples for decades. Our equipment helps you maintain the consistent -70°C environment critical for halting enzymatic degradation and preventing freeze-thaw cycles, ensuring your viral analytes remain stable for future serological and nucleic acid testing.
Contact us today via our contact form to discuss how we can support your biobanking and long-term research goals with tailored lab solutions.
Visual Guide
Related Products
- 58L Precision Laboratory Ultra Low Temperature Upright Freezer for Critical Sample Storage
- 508L Advanced Vertical Ultra Low Temperature Freezer for Critical Laboratory Storage
- 408L Advanced Vertical Laboratory Ultra Low Temperature Freezer for Critical Research Material Preservation
- 108L Vertical Ultra Low Temperature ULT Freezer
- 708L Ultra Low Temperature Freezer High Performance Laboratory Freezer
People Also Ask
- What are ultra-low temperature freezers designed for? Preserving Your Most Valuable Biological Samples
- What features do ultra-low temperature freezers typically include? Ensuring Absolute Sample Security
- What are the recommendations for storing mRNA vaccines in ultra-low temperature freezers? Ensure Absolute Stability at -80°C
- What temperature ranges are typically associated with ultra-low temperature freezers? Preserve Samples from -40°C to -86°C
- What is the role of an ultra-low temperature (ULT) freezer in the freeze-thaw synthesis of hydrogel nanocomposites?



















