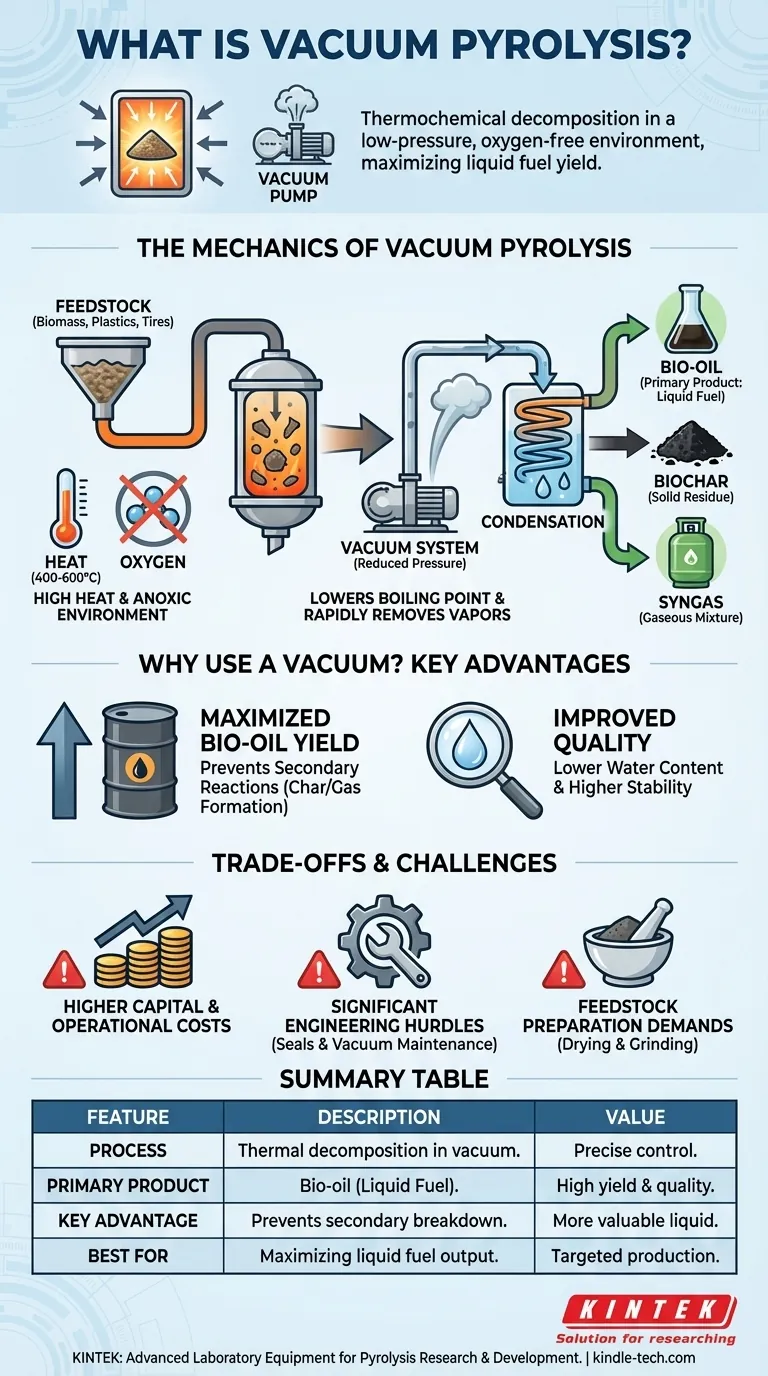
In simple terms, vacuum pyrolysis is a process that uses heat to decompose materials like biomass, plastics, or waste tires in a low-pressure, oxygen-free environment. This thermochemical reaction breaks down large, complex molecules into smaller, more valuable ones, primarily yielding a liquid fuel known as bio-oil.
The critical insight is that the vacuum does more than just remove oxygen; it significantly lowers the boiling point of the decomposition products. This allows them to be rapidly suctioned away as vapor, preventing them from breaking down further into less desirable char and gases, thereby maximizing the liquid oil yield.

The Mechanics of Vacuum Pyrolysis
Vacuum pyrolysis operates on a few core principles that work in concert. Understanding each component reveals why this method is uniquely effective for producing liquid fuels.
The Role of Heat and Anoxia
Like all forms of pyrolysis, the process starts with high heat, typically in the range of 400-600°C. This thermal energy is what breaks the chemical bonds within the feedstock.
Critically, this happens in an anoxic (oxygen-free) environment. The vacuum helps ensure the near-total absence of oxygen, preventing the material from simply burning (combustion) and instead forcing it to decompose.
The Defining Feature: Reduced Pressure
The "vacuum" is what sets this process apart. The reactor is held at a very low pressure, far below normal atmospheric pressure.
This reduced pressure dramatically lowers the boiling point of the volatile compounds released as the feedstock breaks down. They effectively "flash" into a vapor state at temperatures where they would otherwise remain liquid.
The Three Primary Products
The process separates the feedstock into three distinct outputs:
- Bio-oil (or Pyrolysis Oil): A dark, viscous liquid created by cooling and condensing the extracted vapors. This is the primary target product of vacuum pyrolysis.
- Biochar: The solid, carbon-rich residue left behind in the reactor. It resembles charcoal and has applications in agriculture and filtration.
- Syngas (Synthesis Gas): A mixture of non-condensable gases (like hydrogen, carbon monoxide, and methane) that are also produced during decomposition.
Why Use a Vacuum? The Core Advantages
The decision to add the complexity of a vacuum system is driven by the desire to control the chemical reactions with precision, leading to a higher quality and quantity of the desired product.
Maximizing Liquid Bio-oil Yield
The main goal of vacuum pyrolysis is to produce the maximum amount of liquid bio-oil. The vacuum is the key to achieving this.
Preventing Secondary Reactions
In standard pyrolysis, the hot vapors linger in the reactor and can break down further on the surface of the hot char. This secondary cracking turns valuable oil components into non-condensable gas and more char.
The vacuum solves this by instantly removing the vapors from the reactor as they form. They have no time to undergo these secondary reactions, preserving them in their most valuable liquid precursor state.
Improving Bio-oil Quality
Because the vapors are extracted so quickly, the resulting bio-oil often has lower water content and is more chemically stable than oils produced via conventional fast pyrolysis. It contains a higher fraction of valuable compounds.
Understanding the Trade-offs and Challenges
While effective, vacuum pyrolysis is not a universally superior solution. Its advantages come with significant engineering and economic costs.
Higher Capital and Operational Costs
Vacuum systems are inherently complex. They require expensive vacuum pumps, robust seals, and precise instrumentation, leading to higher initial investment compared to atmospheric pyrolysis units.
Furthermore, maintaining a vacuum requires a constant energy input for the pumps, increasing the overall operational cost of the plant.
Significant Engineering Hurdles
Designing a reactor that can hold a vacuum at high temperatures is a major engineering challenge. Preventing air leaks is paramount, as even a small leak can introduce oxygen, compromising the entire process and creating a potential safety hazard.
Feedstock Preparation Demands
To ensure heat transfers rapidly and evenly through the material in a vacuum, the feedstock often needs to be dried and ground into a fine powder. This pre-processing step adds cost and complexity to the overall operation.
Making the Right Choice for Your Goal
Selecting a thermal conversion technology depends entirely on your end-product goal and economic constraints.
- If your primary focus is maximizing the yield of high-quality liquid fuel: Vacuum pyrolysis is a leading technology because it expertly minimizes secondary reactions that degrade the oil.
- If your primary focus is producing biochar for agricultural or filtration use: Atmospheric slow pyrolysis is a far simpler, cheaper, and more direct method.
- If your primary focus is balancing capital cost with speed: Conventional fast pyrolysis offers a compromise, providing high throughput without the added expense and complexity of a full vacuum system.
Ultimately, choosing the right pyrolysis method is about matching the tool to the specific chemical and economic objectives of the project.
Summary Table:
| Feature | Description |
|---|---|
| Process | Thermal decomposition of materials in a vacuum (low-pressure) environment. |
| Primary Product | Bio-oil (liquid fuel), maximized by rapid vapor removal. |
| Key Advantage | Prevents secondary reactions, leading to higher quality and yield of oil. |
| Key Challenge | Higher capital and operational costs due to complex vacuum systems. |
| Best For | Projects where maximizing high-quality liquid fuel yield is the primary goal. |
Ready to transform your biomass or waste materials into high-value products?
At KINTEK, we specialize in advanced laboratory equipment, including pyrolysis systems. Whether you're researching process optimization or scaling up production, the right equipment is critical to your success.
Let our experts help you select the perfect solution for your specific needs. Contact us today to discuss how KINTEK's lab equipment can enhance your pyrolysis research and development.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Lab-Scale Vacuum Induction Melting Furnace
People Also Ask
- Why are SEM samples coated with carbon? For Accurate Elemental Analysis Without Interference
- Why is temperature excursion alarming important in ultra-low freezers? Protect Your Valuable Samples from Catastrophic Loss
- How do infrared thermal imagers or thermocouple monitoring systems evaluate SiC coating thermal oxidation resistance?
- What is the product composition of pyrolysis gas? A Guide to Fuel Composition & Control
- What are the limitations of XRF? Understanding Its Boundaries for Accurate Elemental Analysis
- What temperature does THC distillate evaporate? Master Your Vaping Experience with the Perfect Heat
- What temperature is brazing material? Master the Heat for Perfect Metal Joints
- How do I choose a filter press? Match the Right Technology to Your Slurry for Optimal Results



















