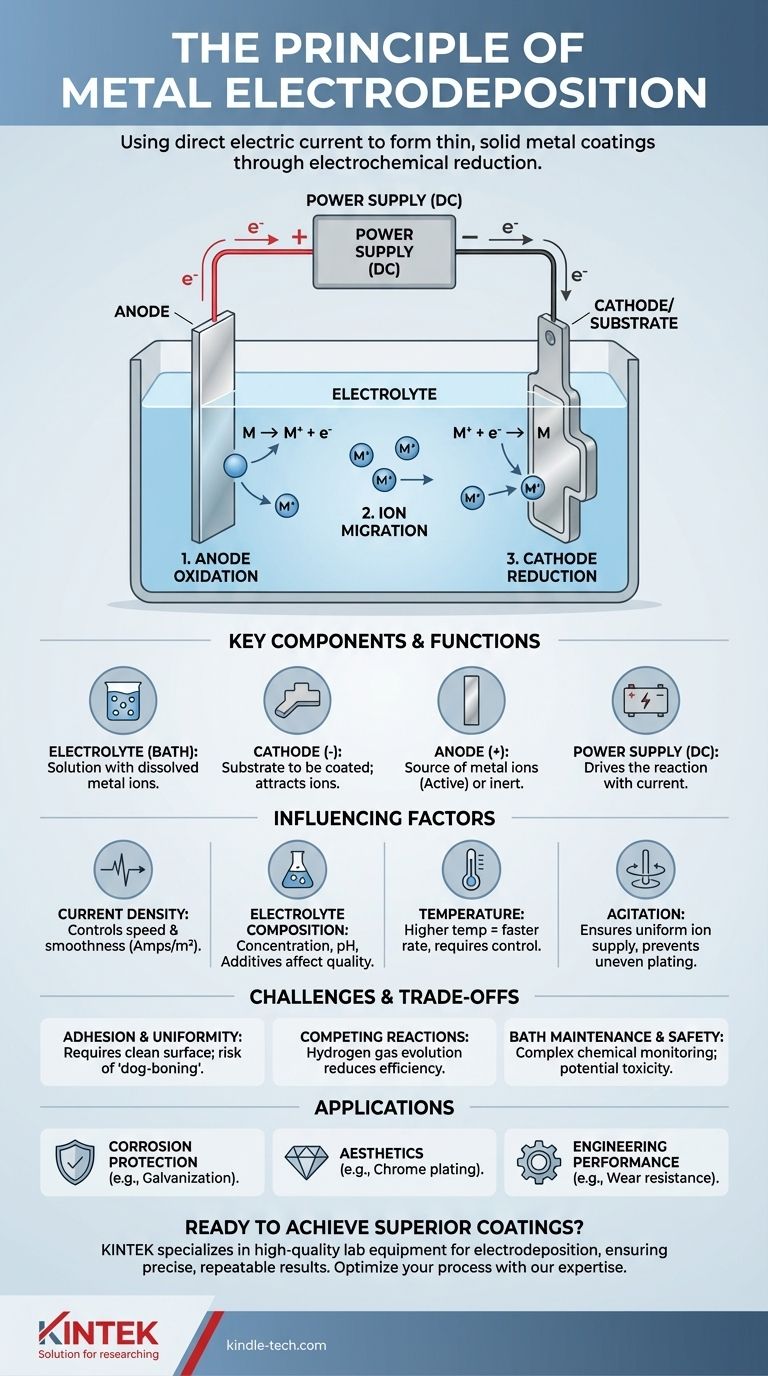
At its core, the principle of electrodeposition is the process of using a direct electric current to reduce dissolved metal ions from a solution and form a thin, solid metal coating onto a conductive object. This controlled electrochemical reaction allows you to "plate" one metal onto another, fundamentally altering the surface properties of the base material.
The entire process hinges on creating an electrolytic circuit. In this circuit, electricity drives a non-spontaneous chemical reaction, forcing positively charged metal ions in a liquid bath to accept electrons and deposit as a neutral metal layer onto a target surface.
The Core Components of an Electrodeposition System
To understand the principle in practice, you must first understand its four essential components working together within an electrolytic cell.
The Electrolyte (The Bath)
The electrolyte is a solution containing a high concentration of the metal ions you wish to deposit. This is typically made by dissolving metal salts (like copper sulfate or nickel chloride) in water. The bath also contains other additives to control the quality of the final coating.
The Cathode (The Substrate)
The cathode is the object you intend to coat. It is connected to the negative terminal of the power supply. This negative charge attracts the positively charged metal ions from the electrolyte.
The Anode (The Metal Source)
The anode is connected to the positive terminal of the power supply. It can be one of two types:
- Active Anode: Made of the same metal being plated. It slowly dissolves, replenishing the metal ions in the electrolyte as they are deposited onto the cathode.
- Inert Anode: Made of a non-reactive material (like platinum or carbon). It does not dissolve but serves to complete the electrical circuit. In this case, the metal ions in the bath are depleted over time.
The Power Supply
A direct current (DC) power supply acts as the engine for the entire process. It provides the electrical potential needed to drive electrons to the cathode and pull them from the anode, forcing the deposition reaction to occur.
The Electrochemical Process, Step-by-Step
The deposition process is a continuous loop of oxidation and reduction driven by the external power supply.
Step 1: Oxidation at the Anode
At the positive anode, an oxidation reaction occurs. If the anode is active, its metal atoms lose electrons and become positively charged ions, dissolving into the electrolyte. This keeps the supply of metal ions consistent.
Step 2: Ion Migration in the Electrolyte
The positively charged metal ions (cations) present in the electrolyte are drawn through the solution toward the negatively charged cathode. Simultaneously, negative ions (anions) drift toward the positive anode, keeping the solution electrically neutral.
Step 3: Reduction at the Cathode
This is the deposition step. When the metal ions reach the cathode, they gain electrons supplied by the power source. This reduction reaction neutralizes their charge, causing them to precipitate out of the solution and bond to the surface as solid metal atoms, building the coating layer by layer.
Key Factors Influencing Deposition Quality
The quality, thickness, and appearance of the final coating are not automatic. They depend on careful control of several key variables.
Current Density
This is the amount of current per unit of surface area of the cathode (measured in amps/m²).
- Low current density results in a slow but often smoother and more uniform coating.
- High current density speeds up deposition but can lead to rough, porous, or burnt deposits if not managed correctly.
Electrolyte Composition
The concentration of metal ions, pH level, and presence of additives (like brighteners and levelers) have a massive impact. These additives can alter the crystal structure of the depositing metal, changing its finish from dull to mirror-bright.
Temperature
Higher bath temperatures generally increase the conductivity of the electrolyte and the rate of deposition. However, excessively high temperatures can cause unwanted side reactions or decomposition of additives.
Agitation
Stirring or otherwise agitating the bath is critical. It ensures a fresh supply of metal ions reaches the cathode surface, preventing localized depletion that causes uneven plating, especially on complex shapes.
Understanding the Trade-offs and Challenges
While powerful, electrodeposition is a precise process with common failure points.
Coating Adhesion and Uniformity
The single most critical factor for success is substrate preparation. An unclean or oxidized surface will result in poor adhesion, causing the coating to peel or flake off. Furthermore, electric current naturally concentrates on sharp edges and corners, leading to thicker deposits there and thinner deposits in recesses—an issue known as the "dog-boning" effect.
Competing Reactions
The primary competing reaction, especially in aqueous electrolytes, is the reduction of water to produce hydrogen gas at the cathode. This process consumes electrical current that would otherwise be used for metal deposition, reducing the overall efficiency. In some cases, absorbed hydrogen can also make the substrate brittle.
Bath Maintenance and Safety
Electrodeposition baths are complex chemical systems that require constant monitoring and adjustment of pH, temperature, and chemical concentrations. Many industrial plating solutions, such as those containing cyanide or hexavalent chromium, are highly toxic and pose significant environmental and operator safety risks.
Making the Right Choice for Your Application
Understanding the core principle allows you to tailor the process to your specific goal.
- If your primary focus is corrosion protection: Your goal is a dense, non-porous layer, often using a sacrificial metal like zinc on steel (galvanization) or a noble metal like gold.
- If your primary focus is aesthetics: You must carefully control current density and use specific additives like brighteners to achieve a smooth, reflective surface, as seen with chrome or nickel plating.
- If your primary focus is engineering performance (e.g., wear resistance): You need precise control over thickness and hardness, often achieved with hard chrome or electroless nickel coatings, where strong adhesion is paramount.
By controlling the flow of ions and electrons, you can transform the surface of a material to meet a specific engineering or aesthetic need.

Summary Table:
| Key Component | Role in Electrodeposition |
|---|---|
| Electrolyte (Bath) | Solution containing dissolved metal ions to be deposited. |
| Cathode (Substrate) | The object to be coated; attracts positive metal ions. |
| Anode (Metal Source) | Source of metal ions (active) or inert electrode. |
| Power Supply (DC) | Provides the current to drive the non-spontaneous reaction. |
| Current Density | Controls deposition speed and coating quality (smoothness). |
| Bath Additives | Influence final coating properties (e.g., brightness, hardness). |
Ready to Achieve Superior Metal Coatings in Your Lab?
Understanding the principles of electrodeposition is the first step. Implementing it effectively requires the right equipment and consumables. KINTEK specializes in high-quality lab equipment for electrodeposition and other surface engineering processes, helping you achieve precise, reliable, and repeatable results.
Whether you are focused on research, quality control, or developing new coatings, our expertise can support your laboratory's needs.
Contact our experts today to discuss how we can help you optimize your electrodeposition process!
Visual Guide
Related Products
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are sputtering systems used for? A Guide to Advanced Thin-Film Deposition
- What is electron beam coating? A Guide to High-Performance PVD Thin Films
- What is the container that holds the metal source material called in e-beam evaporation? Ensure Purity and Quality in Your Thin-Film Deposition
- What is sputter coating used for? Achieve Superior Thin Films for Electronics, Optics, and Tools
- What is gold sputtered? A Guide to High-Purity Vacuum Coating for Electronics & SEM



















