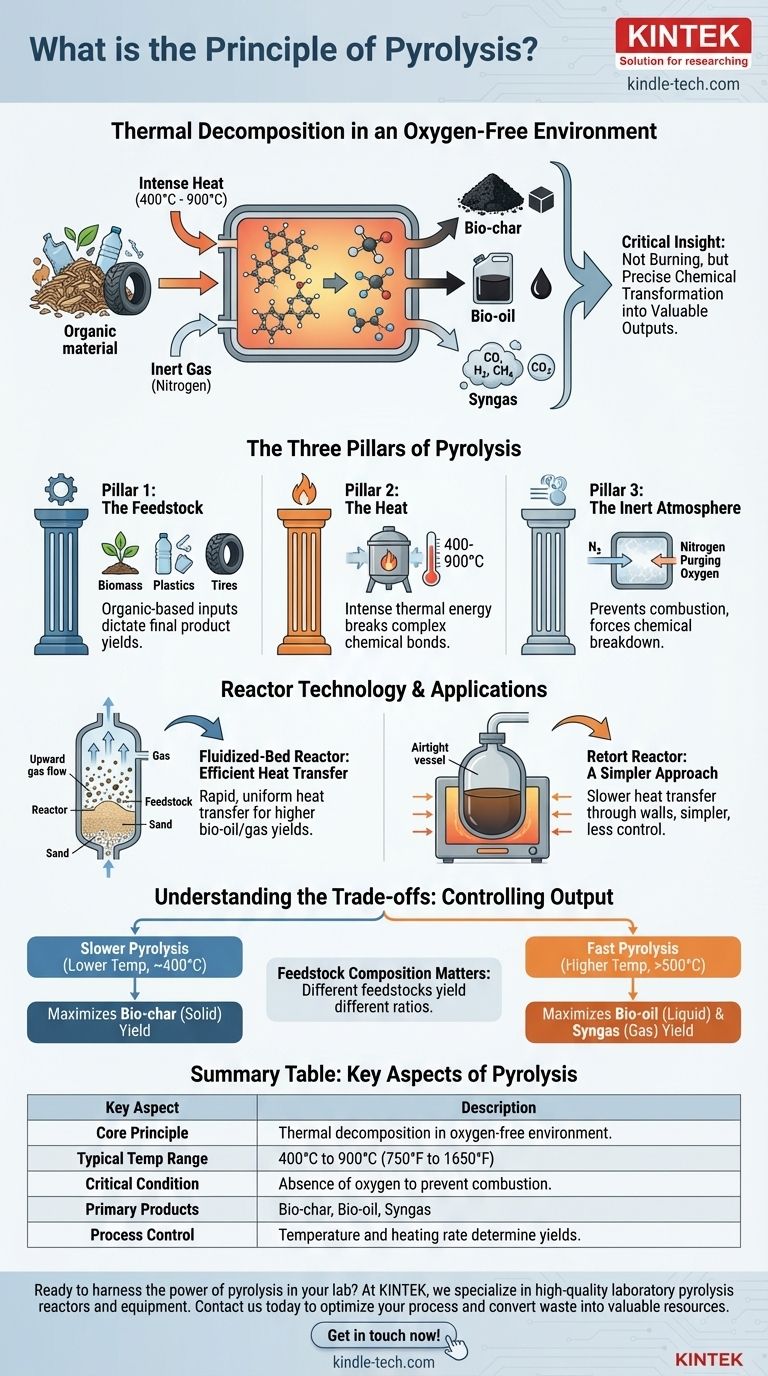
At its core, the principle of pyrolysis is the thermal decomposition of organic material at high temperatures in a strictly controlled, oxygen-free environment. Instead of combusting (burning), the material is chemically broken down by intense heat into a mixture of solid, liquid, and gaseous products.
The critical insight is that pyrolysis is not burning; it is a precise and controlled chemical transformation. By eliminating oxygen and carefully applying heat, it deconstructs complex materials like biomass or plastic into valuable outputs like bio-char, bio-oil, and syngas.

The Three Pillars of Pyrolysis
To fully grasp the principle, it helps to think of pyrolysis as a process resting on three essential pillars: the feedstock, the heat, and the controlled atmosphere.
Pillar 1: The Feedstock
The process begins with an organic-based input material, known as the feedstock. Common examples include biomass (like wood or agricultural waste), plastics, and old tires.
The chemical composition of this feedstock is the primary determinant of the final product yields.
Pillar 2: The Heat
Heat is the engine of pyrolysis. The feedstock is heated inside a vessel called a reactor to temperatures typically ranging from 400°C to 900°C (750°F to 1650°F).
This intense thermal energy breaks the large, complex chemical bonds within the material, causing it to decompose into smaller, more stable molecules.
Pillar 3: The Inert Atmosphere
This is the most defining element of pyrolysis. The process must occur in an environment with little to no oxygen.
This is achieved by sealing the reactor and often purging it with an inert gas, such as nitrogen. Preventing the presence of oxygen is crucial because it stops combustion from occurring, forcing the material to break down chemically rather than simply burn away.
How the Principle is Applied: Reactor Technology
The way heat is applied and the inert atmosphere is maintained depends on the reactor design. Different reactors apply the same core principles in slightly different ways to optimize for certain feedstocks or products.
The Fluidized-Bed Reactor: Efficient Heat Transfer
In this design, the reactor contains a bed of granular material, such as sand. The inert gas (e.g., nitrogen) is pumped up from the bottom.
This gas flow serves two purposes: it creates the required oxygen-free atmosphere and "fluidizes" the sand and feedstock particles, making them behave like a liquid. This constant motion ensures extremely rapid and uniform heat transfer, which often increases the yield of bio-oils and gases.
The Retort Reactor: A Simpler Approach
A retort reactor, sometimes called an auger or kiln, is essentially an airtight vessel heated from an external source, much like an oven.
Heat is transferred more slowly through the vessel's walls to the feedstock inside. This method is mechanically simpler but generally offers less control over the heat transfer rate compared to a fluidized bed.
Understanding the Trade-offs
The final output of a pyrolysis system is not fixed. By manipulating key variables, you can control whether you produce more solid char, liquid oil, or combustible gas.
The Role of Temperature and Heating Rate
The balance between products is highly sensitive to temperature and how quickly the feedstock is heated.
Slower pyrolysis at lower temperatures (around 400°C) provides more time for charcoal-like solids (bio-char) to form, maximizing its yield.
Fast pyrolysis at higher temperatures (above 500°C) with rapid heating rates breaks the material down so quickly that it favors the production of liquids (bio-oil) and gases (syngas).
Feedstock Composition Matters
The inherent chemical makeup of the feedstock plays a significant role. A woody biomass will naturally yield different ratios of oil, gas, and char compared to a feedstock of uniform plastic.
Understanding your feedstock is essential for predicting and optimizing the output of your pyrolysis system.
Tailoring Pyrolysis to Your Goal
The right approach depends entirely on what you want to produce.
- If your primary focus is producing bio-char: Use lower process temperatures and slower heating rates to maximize the formation of a stable, solid carbon structure.
- If your primary focus is producing bio-oil and syngas: Use higher temperatures and a rapid heating method, such as a fluidized-bed reactor, to favor the cracking of molecules into liquids and gases.
By mastering these core principles, you can engineer a pyrolysis process to convert a specific waste stream into a predictable and valuable resource.
Summary Table:
| Key Aspect | Description |
|---|---|
| Core Principle | Thermal decomposition of organic material in an oxygen-free environment. |
| Typical Temperature Range | 400°C to 900°C (750°F to 1650°F) |
| Critical Condition | Absence of oxygen to prevent combustion. |
| Primary Products | Bio-char (solid), Bio-oil (liquid), Syngas (gas) |
| Process Control | Temperature and heating rate determine product yields. |
Ready to harness the power of pyrolysis in your lab?
At KINTEK, we specialize in providing high-quality laboratory pyrolysis reactors and equipment tailored to your research goals. Whether you're focused on producing bio-char, bio-oil, or syngas from biomass or plastic waste, our expertise ensures precise temperature control and optimal performance.
Contact us today to discuss how our solutions can help you optimize your pyrolysis process and convert waste into valuable resources. Get in touch now!
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Test Sieves and Sieving Machines
- Vacuum Dental Porcelain Sintering Furnace
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- How does a sputtering machine work? Achieve Atomic-Level Precision for Your Coatings
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- How does a vacuum oven contribute to solid electrolyte membrane formation? Achieve Dense, Defect-Free Materials
- How mechanical properties are affected by sintering? Master the Trade-offs for Stronger Materials
- What is a sputtering machine? A Guide to High-Quality Thin Film Deposition



















