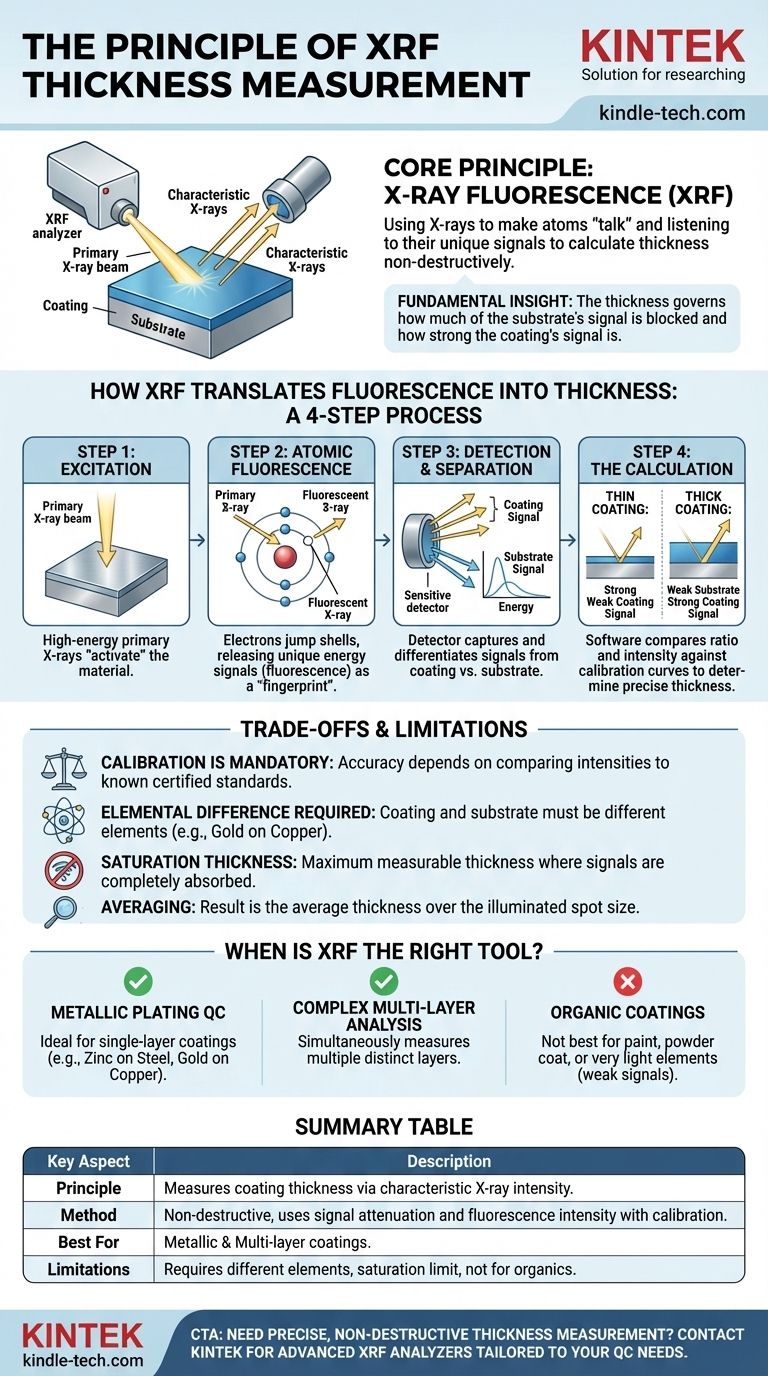
At its core, the principle of X-Ray Fluorescence (XRF) thickness measurement is about using X-rays to make atoms "talk" and then listening to what they say. An XRF analyzer shoots a primary X-ray beam at a coated sample, causing atoms in both the coating and the underlying material (substrate) to emit their own unique, characteristic X-rays. By measuring the intensity of the signals coming back from each layer, the instrument can precisely calculate the thickness of the coating without ever touching it.
The fundamental insight is this: the thickness of a coating directly governs how much of the substrate's signal is blocked and how strong the coating's own signal is. XRF measures this relationship between signals to deliver a fast, non-destructive, and highly accurate thickness reading.

How XRF Translates Fluorescence into Thickness
To understand the principle, it's best to break it down into a sequence of events. Each step is a critical part of a highly controlled physical process.
Step 1: Excitation by Primary X-rays
The process begins when the instrument generates a focused beam of high-energy X-rays. This primary beam is directed onto a small spot on the sample's surface. Think of this beam as the initial energy source that "activates" the material.
Step 2: Atomic Fluorescence
When these primary X-rays strike the sample, they transfer enough energy to knock an electron out of an atom's inner shell. This creates an unstable vacancy.
To regain stability, an electron from a higher-energy outer shell immediately drops down to fill the hole. This transition releases a specific amount of energy in the form of a secondary X-ray, a process called fluorescence.
Crucially, the energy of this fluorescent X-ray is the unique "fingerprint" of the element it came from. A gold atom will emit a different X-ray signal than a copper atom or a zinc atom.
Step 3: Detection and Signal Separation
A highly sensitive detector within the XRF analyzer captures these returning fluorescent X-rays. The analyzer's electronics can differentiate between the energy levels, allowing it to count how many X-rays are coming from the coating material and how many are coming from the substrate material.
Step 4: The Calculation Principle
This is where the measurement happens. The instrument's software analyzes the signal intensities in one of two primary ways:
-
Substrate Signal Attenuation: As the coating gets thicker, it increasingly absorbs the fluorescent X-rays trying to escape from the substrate below. A thin coating allows many substrate signals through, while a thick coating blocks most of them.
-
Coating Signal Intensity: Conversely, the thicker the coating, the more atoms are present to be excited. This results in a stronger fluorescent signal from the coating itself.
By comparing the ratio and intensity of the coating signal versus the substrate signal against pre-loaded calibration curves, the software calculates the exact thickness.
Understanding the Trade-offs and Limitations
While powerful, XRF technology is not a universal solution. Understanding its operational requirements is key to using it effectively.
The Critical Role of Calibration
An XRF analyzer does not measure thickness absolutely. It measures signal intensities and compares them to data from calibration standards—certified samples with known coating thicknesses. Accurate calibration is the foundation of an accurate measurement.
Elemental Difference is Mandatory
XRF relies on being able to distinguish the "fingerprint" of the coating from that of the substrate. Therefore, the coating and substrate must be composed of different elements. You cannot use XRF to measure the thickness of an aluminum coating on an aluminum substrate.
Saturation Thickness
For any given material combination, there is a maximum thickness that XRF can measure. This is called the saturation thickness. Beyond this point, the coating is so thick that it completely absorbs the primary X-rays before they reach the substrate, or it blocks all fluorescent signals from the substrate. The analyzer can only report that the thickness is at or above this limit.
Averaging Over the Spot Size
The measurement result is an average thickness over the area illuminated by the X-ray beam (the "spot size"). This is not an issue for uniform surfaces but can be a factor when measuring small or irregularly shaped components.
When is XRF the Right Tool?
Applying this technology correctly depends entirely on your measurement goal.
- If your primary focus is rapid, non-destructive QC for metallic platings: XRF is the industry standard for measuring single-layer coatings like zinc on steel, gold on copper, or chrome on brass.
- If your primary focus is analyzing complex, multi-layer coatings: Advanced XRF is ideal, as it can simultaneously measure the thickness of multiple distinct layers, such as gold over nickel over a copper base.
- If your primary focus is measuring organic coatings (paint, powder coat) or very light elements: XRF is generally not the best choice, as these materials produce a very weak fluorescent signal. Other methods like eddy current or ultrasonics are often more suitable.
By understanding how XRF uses elemental fingerprints and signal intensity, you can effectively leverage its power for precise quality control.
Summary Table:
| Key Aspect | Description |
|---|---|
| Principle | Measures coating thickness by analyzing the intensity of characteristic X-rays emitted from coating and substrate layers. |
| Method | Non-destructive, based on signal attenuation and fluorescence intensity compared to calibration standards. |
| Best For | Metallic coatings (e.g., zinc on steel, gold on copper), multi-layer coatings. |
| Limitations | Requires different elements for coating/substrate; has a saturation thickness limit; not ideal for organic coatings. |
Need precise, non-destructive thickness measurement for your coatings? KINTEK specializes in lab equipment and consumables, providing advanced XRF analyzers tailored to your laboratory's quality control needs. Our solutions deliver fast, accurate results for metallic and multi-layer coatings, ensuring your products meet the highest standards. Contact us today to find the perfect XRF tool for your application!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Electrolytic Electrochemical Cell for Coating Evaluation
- Custom PTFE Teflon Parts Manufacturer for PTFE Buchner Funnel and Triangular Funnel
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
People Also Ask
- How is the solid-state reaction process conducted for niobate phosphors? Achieve High Phase Purity at 1673 K
- Why is a high-precision co-precipitation apparatus required for Mg-Al-Zn synthesis? Optimize Adsorbent Performance.
- Why does the high-temperature sealing process for inorganic-carbonate dual-phase membranes require a heating furnace with precise temperature control? Ensure Leak-Free Bonds.
- Why is a high-performance laboratory oven required for constant temperature treatment in mineral kinetic studies?
- What is the process of electron beam physical vapor deposition? Achieve High-Purity, High-Melting-Point Coatings
- Is carbon nanotube inhalation toxic? Understanding the Asbestos-Like Risks of Long, Rigid Nanotubes
- What are the applications of thin films in semiconductors? Powering Modern Electronics from Transistors to Solar Cells
- What is the purpose of a mixer? Achieve Perfect Baking Results with Less Effort
















