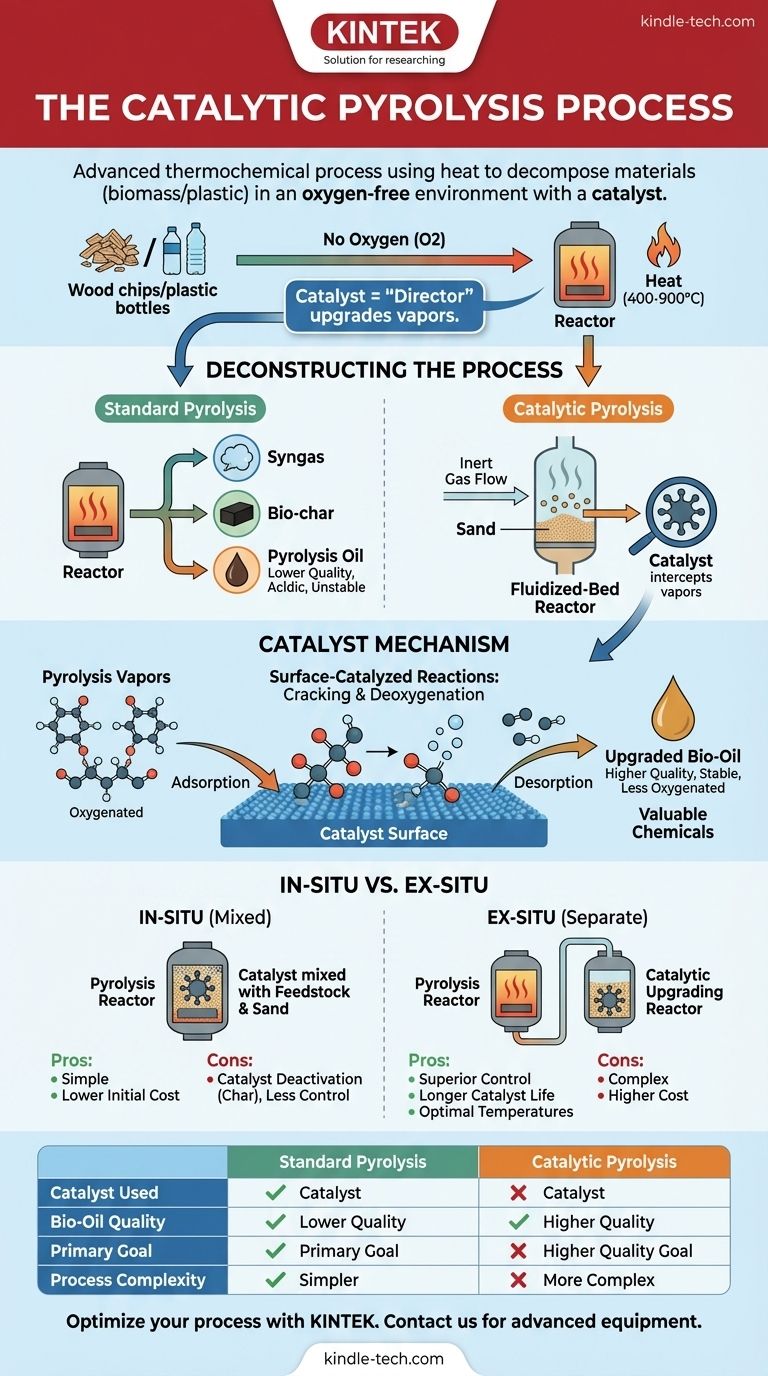
At its core, catalytic pyrolysis is an advanced thermochemical process that uses heat to decompose materials like biomass or plastic in an oxygen-free environment, but with the crucial addition of a catalyst. This catalyst actively upgrades the resulting vapors as they are formed, steering the chemical reactions to produce a higher-quality liquid fuel (bio-oil) and more valuable chemicals.
While standard pyrolysis simply breaks down materials with heat, catalytic pyrolysis introduces a chemical "director"—the catalyst—to intelligently refine the raw outputs into more stable, energy-dense, and valuable products in a single, integrated step.

Deconstructing the Process: From Feedstock to Product
To understand catalytic pyrolysis, we must first understand its foundation and the key components that differentiate it from a standard thermal process.
The Foundation: Standard Pyrolysis
The process begins inside a pyrolysis reactor. Feedstock material, such as wood chips or plastic waste, is heated to high temperatures (typically 400–900°C) in a completely inert, oxygen-free atmosphere.
This intense heat, without oxygen to allow combustion, causes the material's complex molecules to break down, or "decompose." This thermal decomposition yields three primary products: syngas (a mixture of combustible gases), bio-char (a solid, carbon-rich material), and pyrolysis oil (a liquid).
The Reactor Environment: The Fluidized Bed
Many modern pyrolysis plants use a fluidized-bed reactor. This design contains a layer of granular material, such as sand, at the bottom.
An inert gas, usually nitrogen, is continuously pumped up through this bed. This gas flow prevents oxygen from entering and causing unwanted combustion, but it also "fluidizes" the sand particles, causing them to behave like a boiling liquid.
When the feedstock is introduced, this fluidized sand surrounds it, enabling extremely rapid and uniform heat transfer, which is critical for an efficient pyrolysis reaction.
The Key Difference: Introducing the Catalyst
This is where catalytic pyrolysis diverges. The catalyst's role is to intercept the hot vapor stream produced during pyrolysis before it cools and condenses.
These raw vapors contain many large, unstable, and oxygenated molecules that make standard bio-oil acidic, viscous, and difficult to use as a drop-in fuel. The catalyst provides an active surface that promotes chemical reactions to fix these problems on the spot.
How the Catalyst Actually Works
The catalyst is not a passive ingredient; it is the engine of product improvement. Its function is to crack and refine the pyrolysis vapors at a molecular level.
The Molecular Mechanism
The process on the catalyst's surface is a sequence of precise steps. The hot pyrolysis vapors, composed of various gaseous species, are transported to the catalyst.
First, these species are adsorbed onto the active sites on the catalyst's surface. This close contact facilitates surface-catalyzed reactions, primarily breaking down large molecules (cracking) and removing oxygen atoms (deoxygenation).
Once the desired reactions are complete, the new, smaller, and more stable molecules are desorbed from the surface and flow out of the reactor to be condensed into the final, upgraded bio-oil.
The Result: Higher-Quality Bio-Oil
By promoting these reactions, the catalyst dramatically improves the quality of the resulting liquid fuel. The upgraded bio-oil is less acidic, more chemically stable, and has a higher energy content because the undesirable oxygen has been removed.
Understanding the Trade-offs: In-Situ vs. Ex-Situ
The primary strategic decision in designing a catalytic pyrolysis process is where to place the catalyst. This choice has significant implications for performance, cost, and complexity.
The In-Situ Method: Simple but Less Controlled
In an in-situ (or "in place") configuration, the catalyst particles are mixed directly with the feedstock and the sand in the reactor bed.
This is the simplest and often cheapest design. However, the catalyst is directly exposed to bio-char and other contaminants, leading to rapid deactivation. It also forces the pyrolysis and catalytic upgrading to occur at the same temperature, which is often not optimal for both.
The Ex-Situ Method: Complex but More Precise
In an ex-situ (or "out of place") configuration, the process is split into two separate reactors. The first reactor performs standard pyrolysis, and the resulting vapors are then fed into a second, separate reactor bed containing only the catalyst.
This dual-bed system is more complex and expensive, but it offers far greater control. It protects the catalyst from char contamination, extending its life. Most importantly, it allows operators to set the ideal temperature for pyrolysis and a different, ideal temperature for the catalytic upgrading, maximizing both efficiency and product quality.
Making the Right Choice for Your Goal
The choice between these two methods is a classic engineering trade-off between simplicity and precision. Your final decision should be driven by your primary objective.
- If your primary focus is process simplicity and lower initial cost: The in-situ method is the more direct approach, integrating the catalyst directly into the main pyrolysis reactor.
- If your primary focus is maximizing product quality and catalyst lifetime: The ex-situ method offers superior control and catalyst protection, yielding a better final product and more efficient long-term operation.
- If your primary focus is research and process optimization: An ex-situ setup is invaluable for its ability to independently fine-tune the pyrolysis and catalytic upgrading stages.
By understanding these core principles, you can effectively leverage catalytic pyrolysis to transform low-value feedstocks into valuable resources.
Summary Table:
| Aspect | Standard Pyrolysis | Catalytic Pyrolysis |
|---|---|---|
| Catalyst Used | No | Yes (e.g., zeolites) |
| Bio-Oil Quality | Lower, acidic, unstable | Higher, stable, less oxygenated |
| Primary Goal | Basic decomposition | Fuel upgrading & chemical production |
| Process Complexity | Simpler | More complex (in-situ/ex-situ options) |
Ready to optimize your pyrolysis process with precision catalysts and reactors? KINTEK provides advanced lab equipment and consumables tailored for catalytic pyrolysis research and development. Whether you're scaling up biomass conversion or refining plastic waste, our solutions ensure efficient, controlled reactions for superior bio-oil yields. Contact our experts today to explore how we can support your innovation in sustainable energy!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products