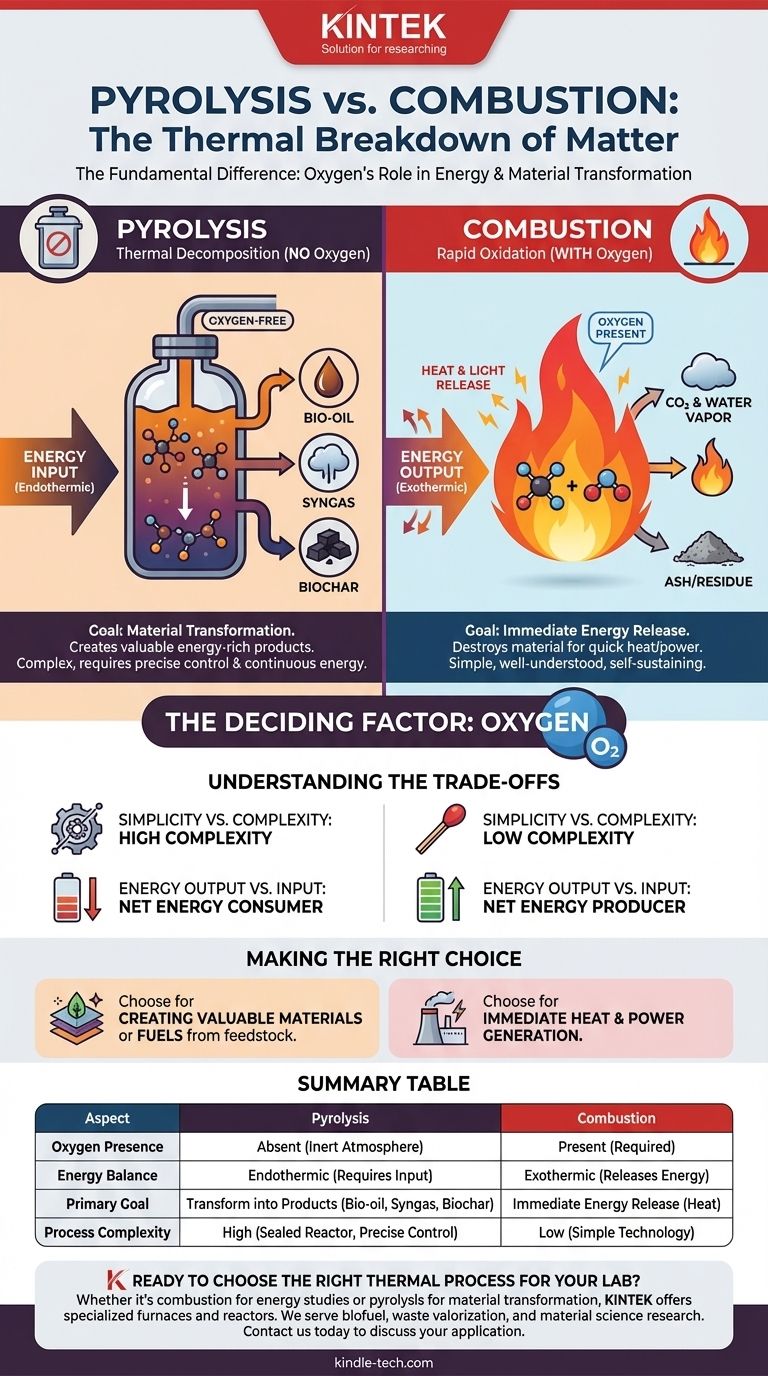
At their core, pyrolysis and combustion are two fundamentally different ways to break down matter with heat. The critical distinction lies in the presence or absence of oxygen. Combustion is a rapid oxidation process—essentially burning—that releases energy, while pyrolysis is a thermal decomposition process that occurs in an oxygen-free environment and requires an input of energy.
The single most important difference is oxygen. Combustion uses oxygen to rapidly release a material's energy as heat, while pyrolysis uses heat in an oxygen-free environment to break a material down into new, energy-rich products.

The Role of Oxygen: The Deciding Factor
The presence or absence of oxygen completely changes the chemical reactions, the energy balance, and the final products of the thermal process.
Combustion: A Reaction with Oxygen
Combustion is what we commonly know as burning. It is an exothermic process, meaning it releases more energy than it consumes, typically in the form of heat and light.
This process involves a fuel reacting rapidly with an oxidizer, which is almost always the oxygen in the air. The outputs are typically simple, low-energy molecules like carbon dioxide and water, along with residual ash.
Pyrolysis: A Decomposition without Oxygen
Pyrolysis is the thermal decomposition of materials at high temperatures in an inert or oxygen-absent atmosphere. Because there is no oxygen to react with, the material does not combust.
Instead, the heat breaks the complex chemical bonds of the feedstock, transforming it into a mix of smaller, often more valuable molecules. This is an endothermic process, meaning it requires a continuous input of energy to sustain the reaction.
Analyzing the Process and Its Products
The goal of each process dictates the value of its outputs. One aims to release energy immediately, while the other aims to store it in new forms.
The Goal of Combustion: Immediate Energy Release
The primary purpose of combustion is to extract the stored chemical energy from a fuel as quickly as possible in the form of usable heat.
The byproducts, such as ash and flue gases, are generally considered low-value waste products that must be managed. The original material is effectively destroyed to release its energy.
The Goal of Pyrolysis: Material Transformation
The primary purpose of pyrolysis is to transform a low-value feedstock into higher-value products. It rearranges the chemical structure of the material rather than just releasing its energy.
The products of pyrolysis—typically a liquid bio-oil, a combustible gas (syngas), and a solid carbon residue (biochar)—all retain significant energy content and have various industrial applications.
Understanding the Trade-offs
Choosing between these processes involves understanding their inherent complexities and energy requirements.
Simplicity vs. Complexity
Combustion is a relatively simple and well-understood technology. Creating a fire requires only fuel, oxygen, and an ignition source.
Pyrolysis is far more complex. It requires a sealed reactor to ensure an oxygen-free environment and precise temperature control, making the equipment more specialized and expensive.
Energy Output vs. Energy Input
Combustion is a net energy producer. Once started, the exothermic reaction sustains itself and releases surplus energy.
Pyrolysis is a net energy consumer. The endothermic process requires a constant and significant external heat source to break down the feedstock. The energy is not lost, but rather stored in the chemical bonds of the new products.
Making the Right Choice for Your Goal
Ultimately, the choice between combustion and pyrolysis depends entirely on what you want to achieve with a given material.
- If your primary focus is immediate heat and power generation: Combustion is the most direct and established method for releasing a fuel's stored energy.
- If your primary focus is creating valuable new materials or fuels from a feedstock: Pyrolysis is the necessary process for transforming matter into energy-dense oils, gases, and char.
Understanding the fundamental role of oxygen is the key to selecting the right thermal process to achieve your specific energy or material goal.
Summary Table:
| Aspect | Pyrolysis | Combustion |
|---|---|---|
| Oxygen Presence | Absent (inert atmosphere) | Present (required for burning) |
| Energy Balance | Endothermic (requires energy input) | Exothermic (releases energy) |
| Primary Goal | Transform feedstock into valuable products (bio-oil, syngas, biochar) | Immediate release of energy as heat |
| Process Complexity | High (requires sealed reactor, precise temperature control) | Low (relatively simple technology) |
Ready to choose the right thermal process for your lab?
Whether you need reliable combustion equipment for energy studies or advanced pyrolysis reactors for material transformation, KINTEK has the solution. Our specialized lab equipment, including furnaces and reactors, is designed for precision, safety, and reproducibility.
We serve laboratories focused on:
- Biofuel and renewable energy research
- Waste valorization and recycling studies
- Material science and chemical analysis
Contact us today to discuss your specific application. Our experts will help you select the ideal equipment to achieve your research or production goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy



















