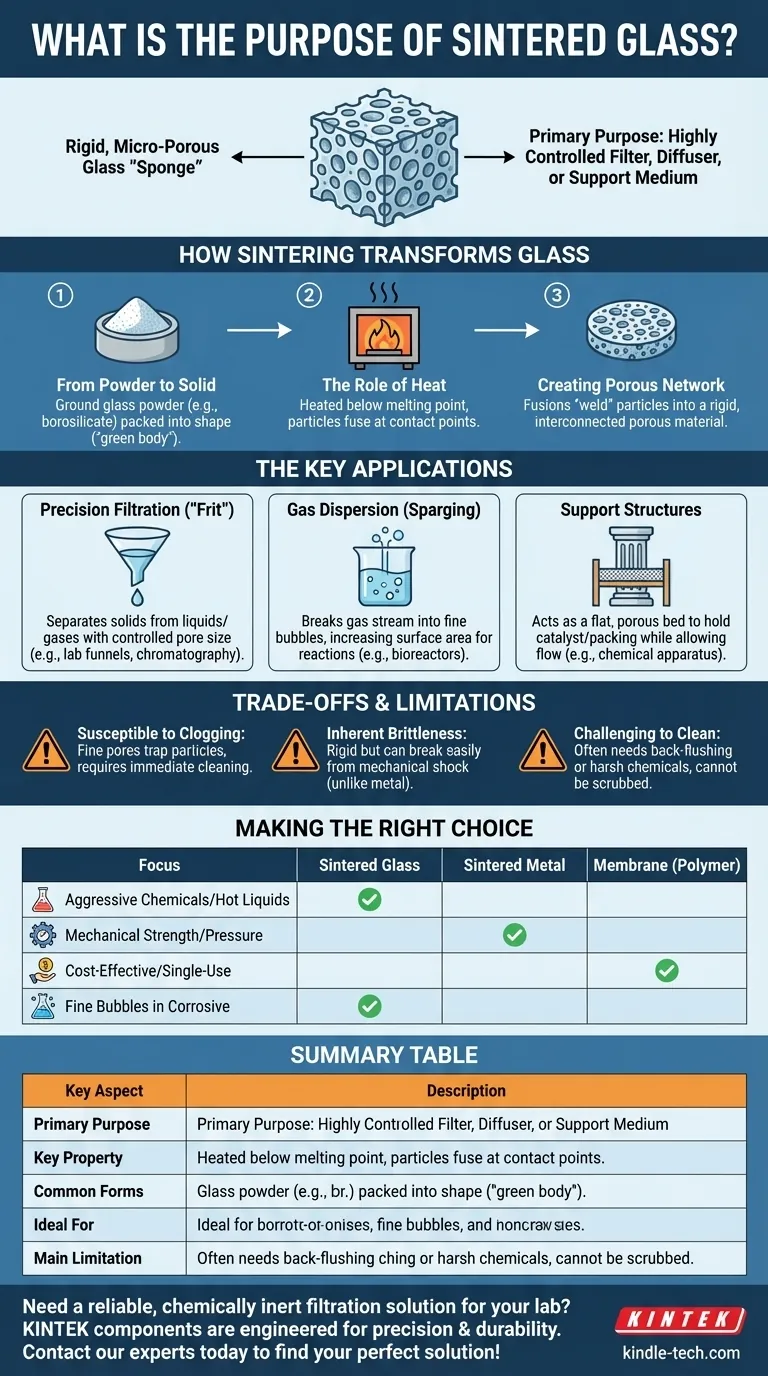
At its core, sintered glass is a specialized material created by fusing glass powder into a solid but porous structure. Its primary purpose is to act as a highly controlled filter, diffuser, or support medium, offering exceptional chemical resistance and thermal stability where other materials would fail. It is an engineered component, not a material for transparency.
Sintered glass is not about creating clear panes; it's about manufacturing a rigid, micro-porous glass "sponge." This unique structure provides a combination of precise filtration, chemical inertness, and the ability to be formed into complex shapes for technical applications.
How Sintering Transforms Glass
From Powder to a Solid Form
The process begins not with molten glass, but with finely ground glass powder, often borosilicate for its chemical and thermal durability. This powder is packed into a mold, sometimes called a "green body," in the desired final shape.
The Role of Heat Without Melting
The packed powder is then heated in a furnace to a temperature below the full melting point of the glass. At this specific temperature, the surfaces of the individual glass particles become soft and fuse together at their points of contact.
Creating a Permanent, Porous Network
This fusion process "welds" the particles into a single, rigid piece. The spaces that existed between the powder grains become a network of interconnected pores, creating the final porous material. The size of these pores is determined by the size of the initial glass particles.
The Key Applications of Sintered Glass
Precision Filtration and Separation
The most common use for sintered glass is as a filter medium, often called a "frit." Because the pore size is highly controlled and consistent, it can be used to separate solids from liquids or gases with high precision. You find it in laboratory funnels, chromatography columns, and industrial filtration systems.
Gas Dispersion (Sparging)
Sintered glass tubes or discs are used as spargers to bubble gas through a liquid. The fine pores break a single gas stream into thousands of tiny bubbles, dramatically increasing the surface area for reactions, absorption, or aeration in chemical reactors and bioreactors.
Support Structures in Chemical Apparatus
In certain laboratory equipment, a sintered glass disc acts as a flat, porous support bed. It can hold a catalyst bed, packing material, or another solid medium in place while allowing liquids or gases to flow through freely.
Understanding the Trade-offs and Limitations
Susceptibility to Clogging
The very fine pores that make sintered glass an excellent filter also make it prone to clogging. Particulate matter or chemical precipitates can become permanently trapped, rendering the filter useless if not cleaned immediately and correctly.
Inherent Brittleness
It is still glass. Sintered glass components are rigid and resistant to heat, but they are brittle and can be easily broken by mechanical shock or by being dropped. This contrasts sharply with more durable metal or polymer filters.
Challenging to Clean
Cleaning a clogged sintered filter is not simple. It often requires back-flushing or soaking in aggressive chemicals like strong acids. Unlike a simple screen, it cannot be physically scrubbed without damaging the delicate porous structure.
Making the Right Choice for Your Application
Choosing the right material depends entirely on the demands of your task.
- If your primary focus is filtering aggressive chemicals or hot liquids: Sintered borosilicate glass is often the superior choice due to its unmatched chemical and thermal resistance.
- If your primary focus is mechanical strength and high-pressure filtration: Sintered metal filters (e.g., stainless steel) offer far greater durability and resistance to pressure shock.
- If your primary focus is cost-effectiveness for single-use applications: Membrane filters made from polymers (like nylon or PTFE) are typically more affordable and disposable.
- If your primary focus is creating fine bubbles in a corrosive liquid: A sintered glass sparger provides excellent performance where a metal or plastic equivalent would degrade.
By understanding sintered glass as an engineered porous material, you can leverage its unique strengths for demanding technical and scientific challenges.

Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Purpose | Acts as a rigid, porous filter, diffuser, or support medium. |
| Key Property | Exceptional chemical resistance and thermal stability. |
| Common Forms | Discs, tubes, and frits for funnels and columns. |
| Ideal For | Filtering aggressive chemicals, gas sparging, catalyst support. |
| Main Limitation | Brittle and prone to clogging if not maintained properly. |
Need a reliable, chemically inert filtration solution for your lab?
Sintered glass components from KINTEK are engineered for precision and durability in demanding applications. Whether you require consistent pore sizes for HPLC analysis, a robust sparger for bioreactors, or a heat-resistant support bed, our borosilicate glass frits deliver unmatched performance.
We specialize in providing the right lab equipment and consumables to enhance your workflow's efficiency and accuracy.
Contact our experts today to find the perfect sintered glass solution for your specific needs!
Visual Guide
Related Products
- Longpass Highpass Filters for Optical Applications
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
- Shortpass Filters for Optical Applications
- Narrow Band Pass Filters for Precision Applications
- Custom PTFE Teflon Parts Manufacturer for Sampling Filters
People Also Ask
- What is the use of thin film in optics? Mastering Light Control for Lenses, Mirrors, and Filters
- What is the acceptable pressure drop across a filter? Master Your System's Health and Efficiency
- What are the advantages of centrifuge filter? Achieve Fast, Gentle Sample Prep for Your Lab
- What is the lifespan of a filter media? Understand the 3 Types for Optimal Filtration
- Why are porous ceramic filters utilized in methane pyrolysis? Protect Your Assets with High-Precision Filtration












