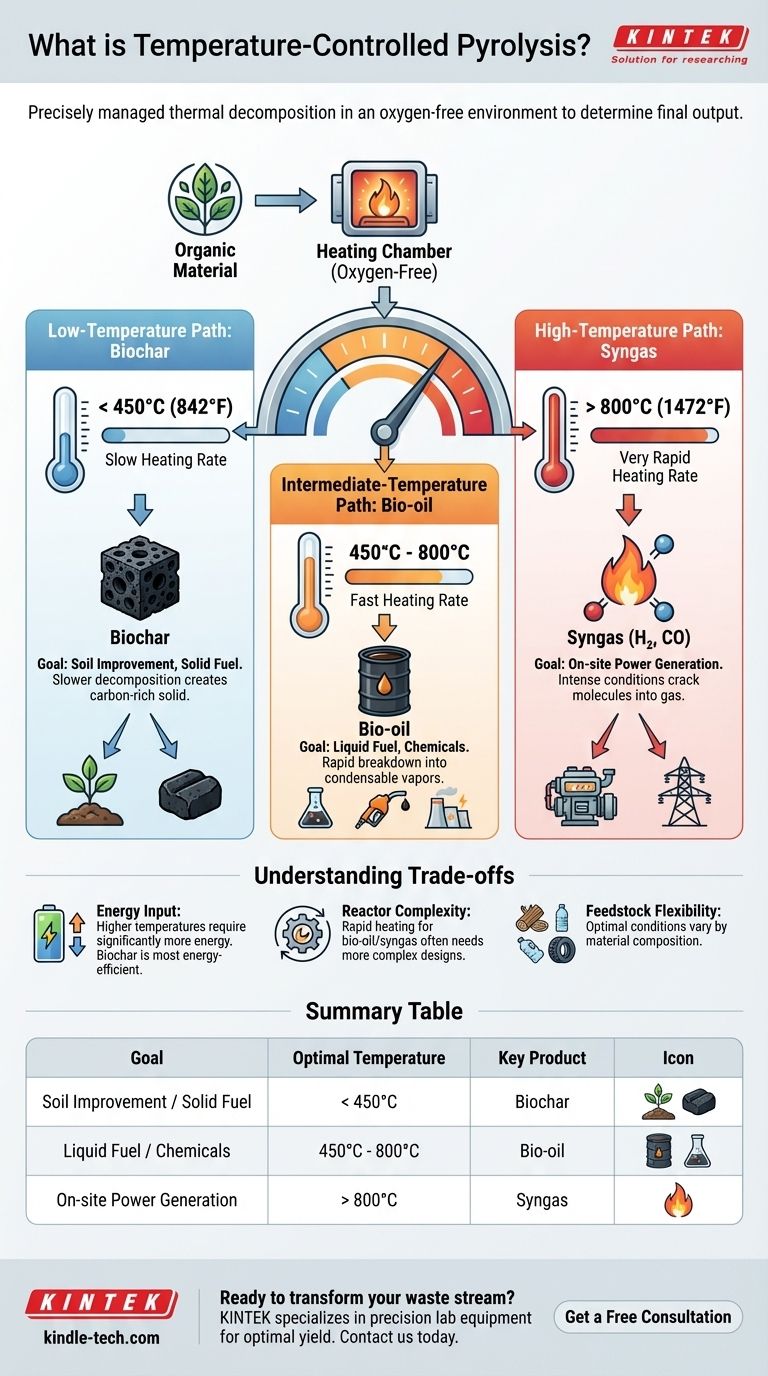
In essence, temperature-controlled pyrolysis is the process of thermally decomposing organic materials in an oxygen-free environment where the temperature and heating rate are precisely managed to determine the final output. It is not just about heating the material; it is about using temperature as a precise tool to dictate whether the primary product will be a solid (biochar), a liquid (bio-oil), or a gas (syngas).
The core principle is simple: controlling the thermal environment is the most critical factor in pyrolysis. Low temperatures favor solid products, high temperatures favor gas, and intermediate temperatures with rapid heating favor liquid fuels. Mastering this control allows you to transform waste into a specific, high-value resource.

The Role of Temperature in Product Formation
Pyrolysis is a conversion technology, and temperature is the primary dial you turn to select the desired conversion. The final temperature, combined with the rate of heating, fundamentally changes the chemical reactions and dictates the state and composition of the end products.
Yielding Biochar: The Low-Temperature Path
At lower temperatures, generally below 450°C (842°F), and with slower heating rates, the process favors the creation of a solid carbon-rich product called biochar.
The slower decomposition allows larger carbon structures to remain intact. This biochar can be used as a potent soil amendment to improve agricultural yields or as a stable, solid fuel similar to coal.
Producing Bio-oil: The Intermediate-Temperature Path
To maximize the yield of liquid bio-oil, a moderate final temperature, often between 450°C and 800°C, is required, combined with a relatively fast heating rate.
These conditions rapidly break down the organic material into smaller, condensable vapors. When cooled, these vapors form a dark, dense liquid bio-oil that can be stored, transported, and used as a furnace fuel, a source for electricity generation, or refined into valuable chemicals.
Generating Syngas: The High-Temperature Path
At very high temperatures, typically above 800°C (1472°F), and with extremely rapid heating, the process is optimized for gas production.
These intense conditions crack the organic molecules into the simplest, non-condensable gaseous components. The resulting product, known as syngas (synthesis gas), is a mixture of hydrogen, carbon monoxide, and other gases that can be immediately used to generate power in gas engines or turbines.
Understanding the Trade-offs
Choosing a temperature is not just a technical decision; it is a strategic one with clear trade-offs in energy consumption, complexity, and final product utility.
Energy Input vs. Product Value
Higher-temperature processes require a significantly greater energy input to maintain. While syngas is a valuable energy source, the operational cost to produce it is higher. Conversely, low-temperature biochar production is the most energy-efficient method.
Heating Rate and Reactor Complexity
Achieving the rapid heating rates necessary for high bio-oil and syngas yields often requires more complex and expensive reactor designs. Slow-heating systems for biochar production can be simpler and less costly to build and operate.
Feedstock Flexibility
The ideal temperature profile can vary depending on the feedstock. Materials like wood, plastics, and tires all have different chemical compositions and will respond differently to the same thermal conditions. Optimizing for a specific waste stream requires fine-tuning the temperature and heating rate.
Making the Right Choice for Your Goal
The optimal pyrolysis temperature is entirely dependent on your primary objective. By defining your goal, you can select the correct thermal conditions to maximize the value of your feedstock.
- If your primary focus is soil improvement or creating a stable solid fuel: Use low temperatures (<450°C) with slow heating rates to maximize your biochar yield.
- If your primary focus is creating a transportable liquid fuel or chemical feedstock: Use intermediate temperatures (450-800°C) with fast heating rates to maximize your bio-oil yield.
- If your primary focus is immediate on-site power generation: Use high temperatures (>800°C) with very rapid heating rates to maximize your syngas yield.
Ultimately, mastering temperature control is what transforms pyrolysis from a simple disposal method into a sophisticated resource-generation technology.
Summary Table:
| Goal | Optimal Temperature | Key Product |
|---|---|---|
| Soil Improvement / Solid Fuel | < 450°C | Biochar |
| Liquid Fuel / Chemicals | 450°C - 800°C | Bio-oil |
| On-site Power Generation | > 800°C | Syngas |
Ready to transform your waste stream into valuable resources? The right pyrolysis equipment is key to achieving your specific product goals. KINTEK specializes in precision lab equipment and reactors for pyrolysis research and development. Our experts can help you select the perfect system to control temperature and heating rates for optimal biochar, bio-oil, or syngas yield. Contact us today to discuss your project and unlock the potential of your materials.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the advantages of catalytic pyrolysis? Produce High-Value Biofuels from Biomass
- How does the calcination process work? Master Thermal Decomposition for Material Purification
- What role does a rotary kiln play in the incineration treatment of waste composite materials? Energy & Volume Solutions
- What is the principle of operation of a rotary kiln? A Guide to Efficient Industrial Thermal Processing
- What is the residence time of pyrolysis? A Key Control Parameter for Bio-oil, Biochar, and Syngas
- How expensive is it to run an electric kiln? Calculate Your True Firing Costs
- What is the chemical reaction of pyrolysis? A Guide to Controlled Thermal Decomposition
- What is the heat source of a rotary kiln? It's a high-intensity burner system.



















