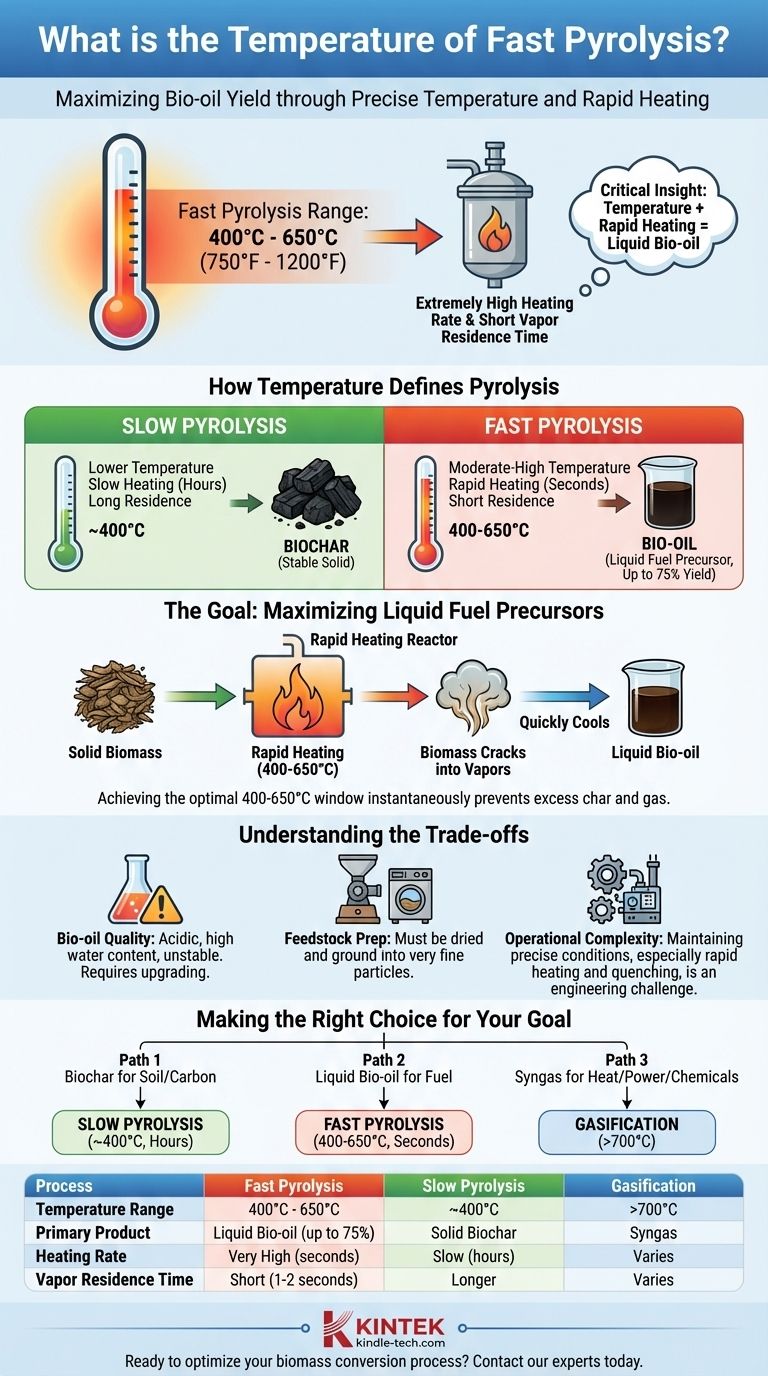
In short, fast pyrolysis typically occurs at moderate to high temperatures, generally within the range of 400°C to 650°C (750°F to 1200°F). However, the defining characteristic of this process is not just the temperature itself, but the extremely high heating rate and short vapor residence time, which are engineered to maximize the production of liquid bio-oil.
The critical insight is that temperature in pyrolysis is a control knob. Fast pyrolysis uses a specific combination of moderate-to-high temperature and rapid heating to intentionally "crack" biomass into liquid bio-oil, fundamentally differing from slower processes that aim to produce solid biochar.

How Temperature Defines the Pyrolysis Process
Pyrolysis is the thermal decomposition of materials in the absence of oxygen. The final output—whether it's a solid, liquid, or gas—is almost entirely determined by the temperature and the rate at which the material is heated.
The Role of Temperature and Heating Rate
Temperature dictates which chemical bonds within the biomass break. A slower process at lower temperatures favors the formation of stable, carbon-rich structures, resulting in biochar.
Conversely, rapid heating to moderate-to-high temperatures causes the biomass polymers (like cellulose and lignin) to fracture violently into smaller, condensable vapors. When cooled quickly, these vapors form a liquid known as bio-oil.
Slow vs. Fast Pyrolysis
Slow pyrolysis involves heating biomass slowly over several hours at lower temperatures, often around 400°C. The primary goal is to maximize the yield of biochar, a stable solid.
Fast pyrolysis, in contrast, heats finely ground biomass to temperatures of 400-650°C in a matter of seconds. This process is specifically designed to maximize the liquid bio-oil yield, which can be up to 75% of the product by weight.
The Goal: Maximizing Liquid Fuel Precursors
The entire purpose of the fast pyrolysis temperature profile is to convert solid biomass into a transportable liquid. While wood pyrolysis can begin at temperatures as low as 200–300°C, this is merely the onset of decomposition.
Achieving the high yields of bio-oil characteristic of fast pyrolysis requires reaching that optimal 400-650°C window almost instantaneously to prevent the formation of excess char and gas.
Understanding the Trade-offs
While fast pyrolysis is an efficient method for creating bio-oil, it comes with specific challenges and considerations that are important to understand.
The Challenge of Bio-oil Quality
The resulting bio-oil is not a drop-in replacement for crude oil. It is acidic, contains a significant amount of water, and can be chemically unstable over time.
This means the bio-oil must be transported to a central facility for significant "upgrading" before it can be used as a conventional fuel, adding complexity and cost to the overall process.
Feedstock Preparation is Crucial
Fast pyrolysis reactors are sensitive to the physical properties of the feedstock. The biomass must be dried to a low moisture content and ground into very fine particles to ensure the rapid heat transfer necessary for the process to work effectively.
Operational Complexity
While the core reactor concept can be simple, maintaining the precise conditions required for fast pyrolysis—especially the rapid heating and subsequent quenching of vapors—is an engineering challenge. This requires sophisticated control systems to ensure consistent product quality and yield.
Making the Right Choice for Your Goal
The optimal temperature and process depend entirely on your desired end product. You must select the thermal conversion strategy that aligns with your specific objective.
- If your primary focus is producing biochar for soil amendment or carbon sequestration: Slow pyrolysis at lower temperatures (around 400°C) over a longer duration is the correct method.
- If your primary focus is maximizing liquid bio-oil for potential fuel production: Fast pyrolysis, with its rapid heating to 400-650°C, is the necessary and most efficient pathway.
- If your primary focus is generating syngas for heat, power, or chemical synthesis: Gasification, which occurs at even higher temperatures (typically above 700°C), is the appropriate technology.
Ultimately, controlling temperature and heating rate provides direct control over the final products of biomass conversion.
Summary Table:
| Process | Temperature Range | Primary Product | Heating Rate | Vapor Residence Time |
|---|---|---|---|---|
| Fast Pyrolysis | 400°C - 650°C | Liquid Bio-oil (up to 75% yield) | Very High (seconds) | Short (1-2 seconds) |
| Slow Pyrolysis | ~400°C | Solid Biochar | Slow (hours) | Longer |
| Gasification | >700°C | Syngas | Varies | Varies |
Ready to optimize your biomass conversion process? KINTEK specializes in laboratory equipment and consumables for pyrolysis research and development. Whether you're scaling up bio-oil production or refining biochar yields, our precise temperature control systems and reactors can help you achieve consistent, high-quality results. Contact our experts today to discuss how we can support your specific laboratory needs and accelerate your renewable energy projects.
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is pyrolysis and the process of pyrolysis? Turn Waste into Valuable Resources
- What is a rotary tube furnace? Achieve Superior Uniformity for Powders and Granules
- What are the benefits of pyrolysis plastic? Transform Waste into Fuel & New Materials
- What are the conditions for pyrolysis of plastic? Key Parameters for Converting Waste into Fuel
- What is the pyrolysis method for plastic waste? Convert Non-Recyclable Plastics into Fuel
- What is the purpose of pyrolysis products? Transform Waste into Valuable Bio-Oil, Bio-Char, and Syngas
- What is the difference between batch and continuous pyrolysis? Choose the Right System for Your Scale
- What is the efficiency of biomass pyrolysis? Maximizing Bio-Oil, Bio-Char, and Syngas Yields



















