The temperature of pyrolysis is not a single number, but rather a critical parameter that is deliberately controlled across a wide range, typically from 300°C to over 700°C (570°F to 1300°F). The specific temperature used depends entirely on the desired end products, as different temperatures favor the creation of gas, liquid bio-oil, or solid biochar. For example, a medium temperature process often operates between 600°C and 700°C.
Pyrolysis temperature is the primary lever used to control the outcome of the process. Choosing the right temperature is a strategic decision that dictates whether you will maximize the production of solid biochar, liquid bio-oil, or flammable gases from your feedstock.

First Principles: What Is Pyrolysis?
The Core Process
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an oxygen-limited or completely oxygen-free environment.
Without oxygen, the material does not combust. Instead, its chemical compounds break down into a mixture of smaller, more valuable molecules.
The Three Key Products
The process transforms a single solid feedstock, like biomass or plastic, into three distinct products:
- Biochar: A stable, carbon-rich solid.
- Bio-oil: A complex liquid mixture of oxygenated hydrocarbons.
- Syngas: A mixture of flammable gases, primarily hydrogen, carbon monoxide, and methane.
The temperature, along with the heating rate, determines the ratio of these three outputs.
How Temperature Dictates Pyrolysis Outcomes
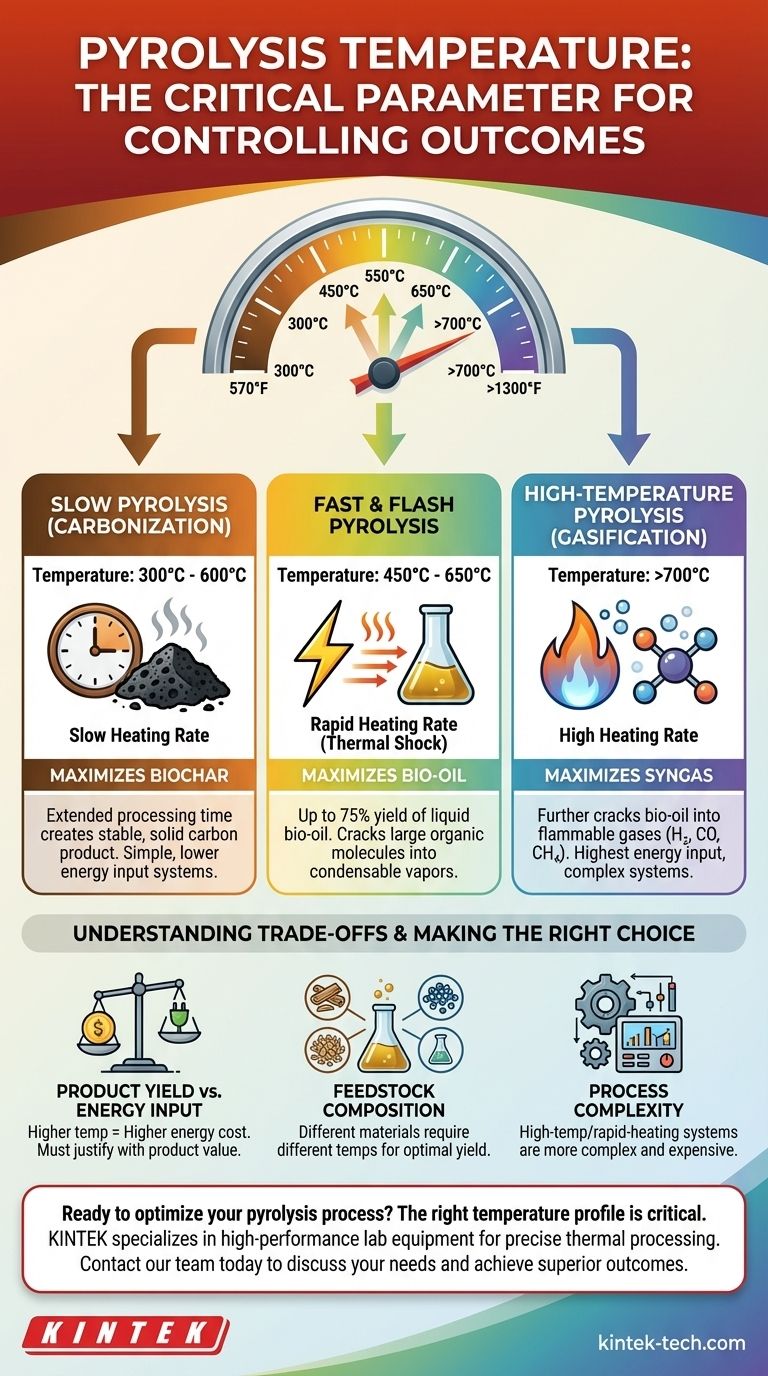
The final temperature and the rate at which it is reached are the most important variables in any pyrolysis system. Different regimes are defined by their unique temperature and heating rate profiles.
Slow Pyrolysis (Carbonization)
Slow pyrolysis uses lower temperatures, typically 300°C to 600°C, and a very slow heating rate (as low as 1°C per minute).
The extended processing time at these lower temperatures maximizes the production of biochar. This process is often called carbonization because its primary goal is to create a stable, solid carbon product.
Fast & Flash Pyrolysis
Fast pyrolysis uses moderate to high temperatures, typically 450°C to 650°C, but with an extremely rapid heating rate. The material is heated to the target temperature in seconds.
This "thermal shock" cracks the large organic molecules into smaller, condensable vapors, maximizing the yield of liquid bio-oil, often reaching up to 75% of the product by weight. Flash pyrolysis is an even more extreme version of this.
High-Temperature Pyrolysis (Gasification)
When temperatures exceed 700°C, the process begins to favor the production of syngas. At these high temperatures, the longer hydrocarbon chains from the bio-oil are further cracked into very simple, non-condensable gas molecules.
This range aligns with the "medium temperature pyrolysis" of 600-700°C mentioned in reference material, which serves as a transition zone where both liquid and gas production are significant.
Understanding the Trade-offs
Choosing a pyrolysis temperature is an engineering decision that requires balancing competing priorities. There is no universally "best" temperature, only the best temperature for a specific goal.
Product Yield vs. Energy Input
Higher temperatures require a significantly greater energy input to maintain. Running a system at 800°C is far more costly than at 450°C.
This cost must be justified by the value of the desired product. If syngas for electricity generation is the goal, the high energy cost may be acceptable. If biochar is the goal, a high-temperature process is inefficient.
Feedstock Composition
Different feedstocks break down at different temperatures. For example, woody biomass and plastics have different chemical compositions and will respond differently to the same heat profile.
Optimizing a process requires tuning the temperature to the specific material being processed to achieve the highest-quality output and yield.
Process Complexity
High-temperature and rapid-heating systems are generally more complex and expensive to build and operate. They require more sophisticated reactors and heat exchangers.
In contrast, slow pyrolysis systems for biochar production can be simpler in design, making them more accessible for smaller-scale or decentralized applications.
Making the Right Choice for Your Goal
To select the correct temperature, you must first define your primary objective.
- If your primary focus is producing biochar for agriculture or carbon sequestration: Use slow pyrolysis with lower temperatures (300-600°C) and slow heating rates.
- If your primary focus is producing liquid bio-oil for renewable fuels or chemicals: Use fast pyrolysis with moderate temperatures (450-650°C) and extremely rapid heating.
- If your primary focus is generating syngas for heat or power: Use high-temperature pyrolysis or gasification (above 700°C) to maximize gas yield.
Ultimately, temperature is the most powerful tool you have to steer the pyrolysis reaction toward the products you value most.
Summary Table:
| Pyrolysis Type | Temperature Range | Primary Product | Key Feature |
|---|---|---|---|
| Slow Pyrolysis | 300°C - 600°C | Biochar | Maximizes solid carbon yield |
| Fast Pyrolysis | 450°C - 650°C | Bio-oil | Maximizes liquid yield (up to 75%) |
| High-Temperature | >700°C | Syngas | Maximizes gas production |
Ready to optimize your pyrolysis process? The right temperature profile is critical for maximizing your yield of biochar, bio-oil, or syngas. KINTEK specializes in high-performance lab equipment and consumables for precise thermal processing. Our experts can help you select the ideal system for your specific feedstock and target products. Contact our team today to discuss your laboratory needs and achieve superior pyrolysis outcomes.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What kind of heat transfer occurs in a vacuum or empty space? Unlocking the Secrets of Thermal Radiation
- What is the brazing process? A Guide to Strong, Permanent Metal Joining
- What critical processing conditions do high-temperature industrial furnaces provide for P91 PWHT? Ensure Joint Integrity
- What are the disadvantages of air quenching? Slow Cooling Limits Hardness and Material Choice
- What is the purpose of constant temperature heating equipment in in-situ curing? Optimize Quasi-Solid-State Electrolytes
- What is the working principle of heat treatment furnace? A Guide to Controlled Material Transformation
- How thick of metal can you braze? Mastering Heat Management for Strong Joints
- What is the overview of vacuum arc remelting? Achieve Ultra-Clean, High-Performance Alloys



















