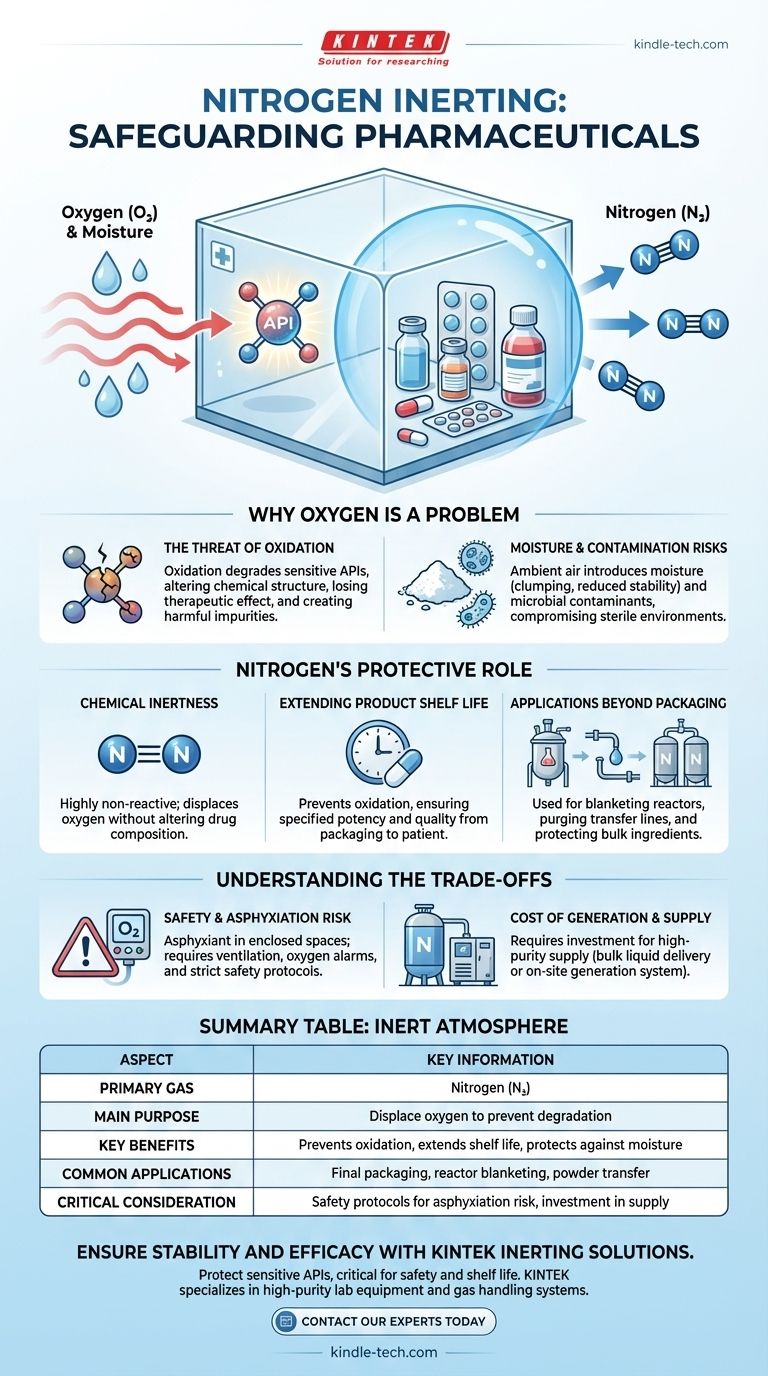
In the pharmaceutical industry, nitrogen is the primary gas used to create an inert atmosphere. This process, often called nitrogen blanketing or inerting, involves displacing oxygen from packaging and processing environments. Doing so protects sensitive active pharmaceutical ingredients (APIs) and finished products from degradation, ensuring their stability, efficacy, and shelf life.
The core challenge is not just packaging, but protecting sensitive chemical compounds from oxygen and moisture at every stage of production. Using an inert gas like nitrogen is the industry-standard solution for preventing oxidative degradation, which is a primary cause of drug spoilage and loss of potency.

Why Oxygen is a Problem in Pharmaceuticals
Oxygen is highly reactive and poses a significant threat to the stability of many pharmaceutical products. Understanding its specific risks clarifies why inerting is a critical, non-negotiable step in manufacturing.
The Threat of Oxidation
Oxidation is a chemical reaction that degrades sensitive compounds. When an Active Pharmaceutical Ingredient (API) oxidizes, its chemical structure changes, which can lead to a loss of therapeutic effect.
This degradation can also create harmful impurities, compromising the safety and quality of the final drug product.
Moisture and Contamination Risks
Ambient air contains moisture. For many powdered or lyophilized (freeze-dried) drugs, exposure to moisture can cause clumping, reduce stability, and accelerate degradation.
Furthermore, using ambient air can introduce microbial contaminants or other particulates, which is unacceptable in the sterile environment required for pharmaceutical production.
Nitrogen's Role as a Protective Barrier
Nitrogen (N₂) is the ideal choice for creating an inert atmosphere due to its chemical properties and availability. It acts as a reliable, invisible shield throughout the manufacturing process.
Chemical Inertness
Nitrogen gas consists of two nitrogen atoms bonded by a powerful triple bond. This bond is extremely difficult to break, making the molecule highly non-reactive, or inert.
Because it doesn't readily react with other chemicals, it can displace oxygen without altering the drug's composition. It effectively "blankets" the product, protecting it from unwanted chemical reactions.
Extending Product Shelf Life
By preventing oxidation, nitrogen blanketing is one of the most effective methods for extending the shelf life of a drug. It ensures the product maintains its specified potency and quality from the moment it's packaged until it reaches the patient.
This is especially critical for liquid medications, injectables, and any API known to be sensitive to oxygen.
Applications Beyond Packaging
Nitrogen's role isn't limited to the final packaging step. It is used throughout the production process to maintain an inert environment.
This includes blanketing chemical reactors during synthesis, purging transfer lines to move sensitive materials, and protecting bulk ingredients stored in tanks or silos.
Understanding the Trade-offs
While nitrogen is the industry standard, implementing an inerting system requires careful consideration of safety and cost. It is not a trivial undertaking.
Safety and Asphyxiation Risk
Nitrogen is not toxic, but it is an asphyxiant. By displacing oxygen, high concentrations of nitrogen in an enclosed space can create an environment that cannot support life.
Proper ventilation, oxygen monitoring alarms, and strict safety protocols are mandatory in any facility where nitrogen is used to prevent accidental asphyxiation of personnel.
Cost of Generation and Supply
Pharmaceutical-grade nitrogen must be extremely pure. While nitrogen is abundant in the atmosphere (about 78%), separating it to the required purity has a cost.
Facilities must choose between having bulk liquid nitrogen delivered in tanks or investing in an on-site nitrogen generation system. The choice depends on consumption volume, purity requirements, and capital expenditure budgets.
Making the Right Choice for Your Process
Applying an inert atmosphere is about mitigating specific risks. Your strategy should be tailored to the sensitivity of your product and the stage of production.
- If your primary focus is final product stability: Your key action is to implement nitrogen flushing in your blister packs, vials, or bottles during the final packaging stage.
- If your primary focus is API integrity during synthesis: Your key action is to blanket the headspace of reactors and storage tanks with nitrogen to prevent side reactions and degradation.
- If your primary focus is preventing contamination during transfer: Your key action is to use pressurized nitrogen to purge and move powders or liquids through transfer lines instead of using compressed air.
Ultimately, integrating a nitrogen inerting system is a fundamental practice for ensuring pharmaceutical products are safe, effective, and stable.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Gas | Nitrogen (N₂) |
| Main Purpose | Displace oxygen to prevent degradation of APIs and finished products. |
| Key Benefits | Prevents oxidation, extends shelf life, protects against moisture/contamination. |
| Common Applications | Final packaging (vials, blisters), reactor blanketing, powder transfer. |
| Critical Consideration | Requires safety protocols for asphyxiation risk and investment in supply/generation. |
Ensure the stability and efficacy of your pharmaceutical products with reliable inerting solutions from KINTEK.
We understand that protecting sensitive APIs from oxidation is critical to your product's safety and shelf life. KINTEK specializes in high-purity lab equipment and gas handling systems tailored to the stringent needs of pharmaceutical laboratories and production facilities.
Let us help you design and implement the right nitrogen blanketing or inert atmosphere system for your specific process—from R&D to full-scale manufacturing. Contact our experts today to discuss your requirements and safeguard your valuable products.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality
- What is an inert condition? A Guide to Preventing Fires and Explosions
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes



















