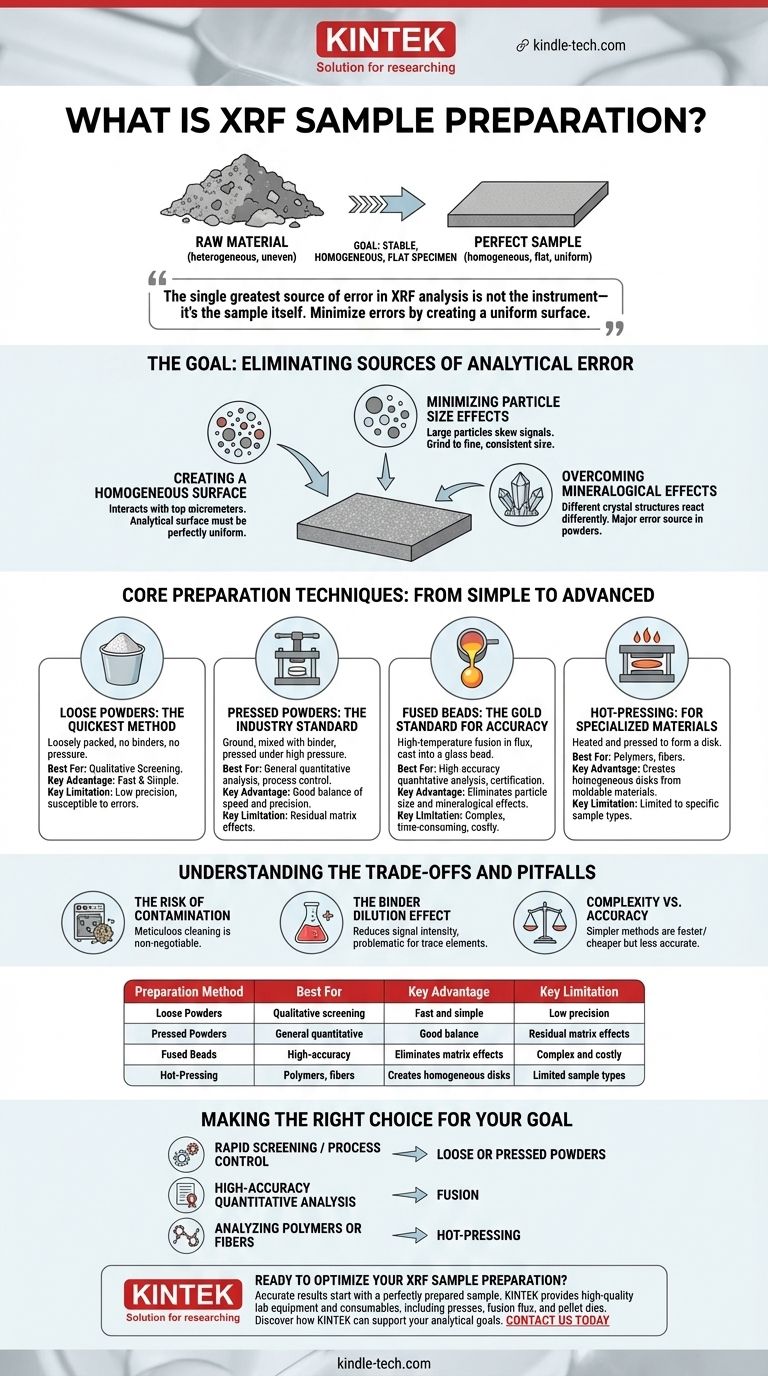
At its core, X-Ray Fluorescence (XRF) sample preparation is the process of transforming a raw material into a stable, homogeneous, and flat specimen suitable for analysis. This crucial step is not just a preliminary task but the most significant factor influencing the accuracy, precision, and reliability of your final analytical results. Without proper preparation, even the most advanced spectrometer will produce questionable data.
The single greatest source of error in XRF analysis is not the instrument—it's the sample itself. The fundamental goal of preparation is to minimize these sample-related errors by creating a perfectly uniform surface that is truly representative of the bulk material.

The Goal: Eliminating Sources of Analytical Error
The XRF instrument analyzes a very small and shallow portion of the sample surface. If that surface is not perfectly representative of the entire sample, the results will be inaccurate. Proper preparation aims to solve three fundamental problems.
Creating a Homogeneous Surface
The X-ray beam interacts with the top few micrometers of the sample. To get an accurate reading of the bulk material, this analytical surface must be perfectly homogeneous, meaning its composition is uniform throughout.
Minimizing Particle Size Effects
If a sample powder contains particles of varying sizes, the X-ray signal can be skewed. Large particles can create micro-shadows or disproportionately absorb or fluoresce X-rays, leading to unreliable measurements. Grinding the sample to a fine, consistent particle size is critical to prevent this.
Overcoming Mineralogical Effects
Two samples can have the exact same elemental composition but different crystal structures (minerals). This difference can cause them to interact with X-rays differently, producing varying results. This is known as the mineralogical effect, and it is a major source of error in powder analysis.
Core Preparation Techniques: From Simple to Advanced
The method you choose depends on your sample type, the elements you are analyzing, and the level of accuracy required. The techniques range from simple mechanical processes to more complex chemical dissolution.
Loose Powders: The Quickest Method
This is the simplest technique, involving loosely packing a finely ground powder into a sample cup. It requires no chemical binders or high pressure.
While fast and easy, it is the least precise method. It is highly susceptible to errors from particle size, inconsistent density, and surface irregularities. It is best used for qualitative screening, not high-accuracy quantitative work.
Pressed Powders: The Industry Standard
This is the most common method for analyzing powders. The sample is ground to a fine powder, mixed with a binder (like a cellulose-wax blend), and pressed under high pressure to form a solid, durable pellet.
The binder helps particles stick together, creating a stable and flat analytical surface. While pressed pellets are far more reliable than loose powders, they still suffer from residual particle size and mineralogical effects.
Fused Beads: The Gold Standard for Accuracy
Fusion is a high-temperature method where the sample is completely dissolved in a molten solvent, typically a lithium borate flux. This is done in a platinum crucible.
The molten mixture is then cast into a mold to form a perfectly homogeneous glass disk, or "bead." This process is destructive but completely eliminates both particle size and mineralogical effects, as the original sample structure is gone. It is the required method for the highest level of accuracy and precision.
Hot-Pressing: For Specialized Materials
For certain materials like hot-moldable polymers (PE, PP) or fibers, hot-pressing is used. The material is heated to a specific temperature and pressed to form a solid, homogeneous disk. This method is specific to materials that can be reshaped with heat and pressure.
Understanding the Trade-offs and Pitfalls
Choosing a preparation method involves balancing speed, cost, and accuracy. Every technique has potential downsides that must be managed to ensure data integrity.
The Risk of Contamination
Contamination is a primary enemy of accurate analysis. It can be introduced from grinding equipment, spatulas, or through cross-contamination from previously prepared samples. Meticulous cleaning of all equipment is non-negotiable.
The Binder Dilution Effect
When creating pressed powders, the addition of a binder (often 20-30% by weight) dilutes the original sample. This reduces the signal intensity for all elements, which can be particularly problematic when measuring trace elements or very light elements.
Complexity vs. Accuracy
The trade-off is clear: simpler methods are faster and cheaper but less accurate.
- Loose Powder: Fastest, but lowest precision.
- Pressed Powder: Good balance, but susceptible to matrix effects.
- Fused Bead: Most complex and expensive, but delivers the highest accuracy by eliminating matrix effects.
Making the Right Choice for Your Goal
Your analytical objective determines the correct preparation strategy. There is no single "best" method, only the one that is most appropriate for your specific application and accuracy requirements.
- If your primary focus is rapid screening or simple process control: Loose or pressed powders provide a sufficient balance of speed and reasonable precision.
- If your primary focus is high-accuracy quantitative analysis for certification or research: Fusion is the required method to eliminate matrix effects and achieve reliable results.
- If your primary focus is analyzing polymers or fibers: Hot-pressing is the specialized technique designed to create uniform disks from these materials.
Ultimately, investing time in a consistent and appropriate sample preparation procedure is the single most important step toward generating data you can trust.
Summary Table:
| Preparation Method | Best For | Key Advantage | Key Limitation |
|---|---|---|---|
| Loose Powders | Qualitative screening, rapid analysis | Fast and simple | Low precision, susceptible to errors |
| Pressed Powders | General quantitative analysis, process control | Good balance of speed and precision | Residual matrix effects |
| Fused Beads | High-accuracy quantitative analysis, certification | Eliminates particle size and mineralogical effects | Complex, time-consuming, and costly |
| Hot-Pressing | Polymers, fibers, specialized materials | Creates homogeneous disks from moldable materials | Limited to specific sample types |
Ready to Optimize Your XRF Sample Preparation?
Accurate results start with a perfectly prepared sample. KINTEK specializes in providing high-quality lab equipment and consumables tailored for XRF analysis, including presses, fusion flux, and pellet dies. Our expertise ensures your laboratory achieves the highest levels of precision and reliability.
Contact us today to discuss your specific needs and discover how KINTEK can support your analytical goals with trusted solutions.
Visual Guide
Related Products
- Three-dimensional electromagnetic sieving instrument
- Laboratory Hybrid Tissue Grinding Mill
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Laboratory Vortex Mixer Orbital Shaker Multifunctional Rotation Oscillation Mixer
- Vibratory Sieve Shaker Machine Dry Three-Dimensional Vibrating Sieve
People Also Ask
- What are the applications of sieving machine? From Mining to Pharmaceuticals
- What is powder sieving? A Guide to Accurate Particle Size Separation
- What are the different types of sieving machines? Choose the Right Motion for Your Material
- What are the components of a sieving machine? Unlock the Anatomy of Precision Particle Separation
- What is the speed of a sieving machine? Optimize Vibration for Maximum Efficiency and Accuracy



















