For X-ray fluorescence (XRF) analysis, the focus is less on a specific required volume or weight and more on the sample's form and surface quality. The most critical requirement is presenting a perfectly flat, smooth, and homogeneous surface to the instrument's X-ray beam, as this ensures the distance to the source and detector remains constant and the results are representative of the whole sample.
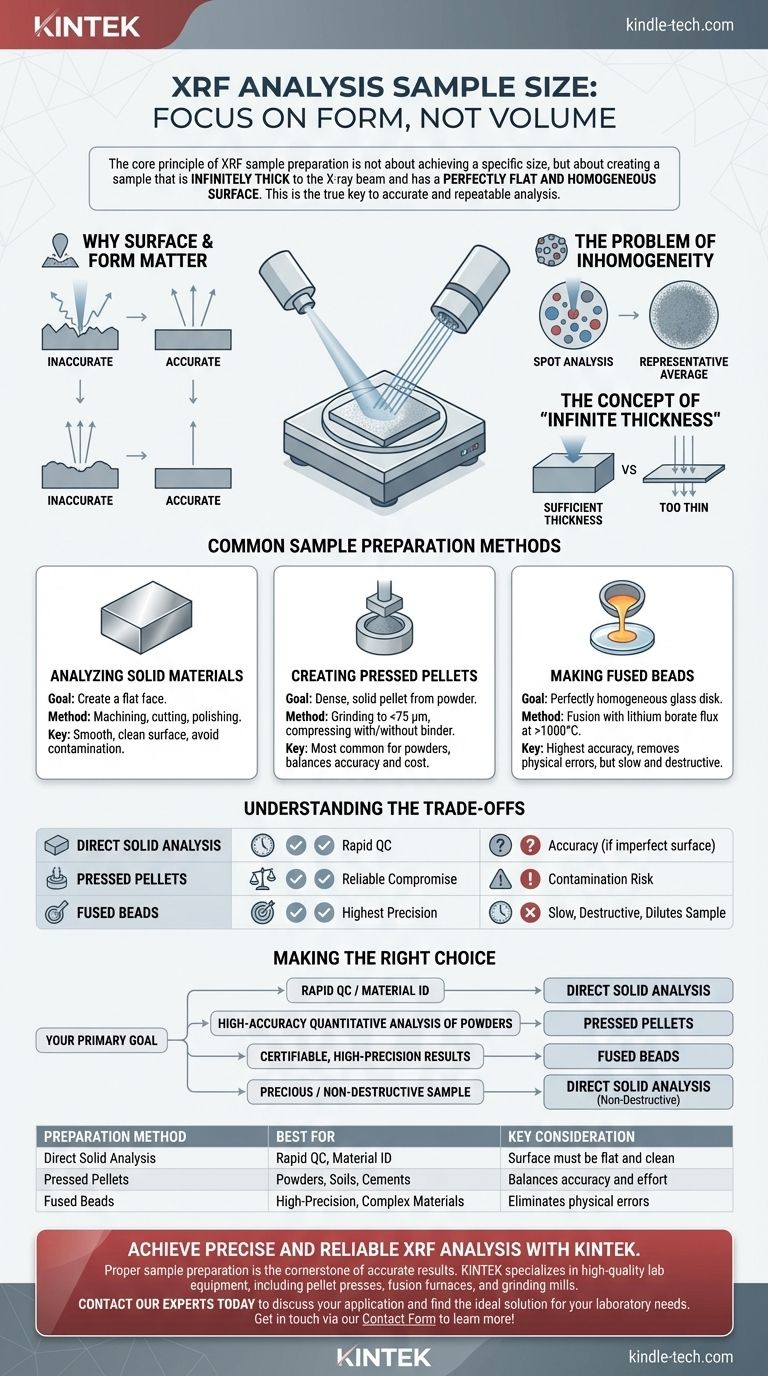
The core principle of XRF sample preparation is not about achieving a specific size, but about creating a sample that is infinitely thick to the X-ray beam and has a perfectly flat and homogeneous surface. This is the true key to accurate and repeatable analysis.

Why Surface and Form Matter More Than Size
The quality of your XRF results is directly tied to how the sample is presented to the analyzer. An improperly prepared sample, regardless of its size, will produce unreliable data.
The Critical Role of Sample Distance
XRF analyzers are calibrated for a precise distance between the X-ray source, the sample surface, and the detector.
If a sample surface is irregular, curved, or rough, different points on the surface will be at different distances from the analyzer. This variation directly alters the intensity of the fluorescent X-rays detected, introducing significant errors into your quantitative results.
The Problem of Inhomogeneity
XRF analyzes a specific spot on the sample surface. If the material is not uniform (homogeneous), the analysis will only reflect the composition of that small spot, not the bulk material.
This is why powdered samples are ground to a very fine, consistent grain size (<75 µm). This process ensures that the portion being analyzed is a statistically representative average of the entire sample.
The Concept of "Infinite Thickness"
For accurate analysis, the sample must be "infinitely thick." This doesn't mean it needs to be enormous; it means it must be thick enough to completely absorb the primary X-ray beam.
If the sample is too thin, the X-rays can pass through it, and the results will be skewed. The required thickness depends on the sample's density and the energy of the X-rays, but for most materials, a few millimeters is sufficient.
Common Sample Preparation Methods
Your preparation method will depend on whether your sample is a solid, a powder, or a liquid. Each method aims to create that ideal flat and homogeneous surface.
Analyzing Solid Materials
For solid metal or polymer samples, the goal is to create a flat face for analysis. This is often done by machining, cutting, or polishing the sample.
The surface must be smooth and clean. It is critical to avoid contamination from polishing materials or tools used on other sample types.
Creating Pressed Pellets
This is the most common method for powders, soils, and cements. The sample is ground into a fine powder and then compressed under high pressure in a die to form a dense, solid pellet.
Sometimes, a wax or cellulose binder is mixed with the powder to help it form a durable pellet that won't crumble during analysis. This method provides excellent results at a relatively low cost.
Making Fused Beads
For the highest level of accuracy, especially with geological samples, fusion is used. The powdered sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C until it melts.
The molten glass is then cast into a mold to create a perfectly homogeneous, flat glass disk. This eliminates mineralogical and particle size effects but is a more complex and time-consuming process.
Understanding the Trade-offs
Choosing a preparation method involves balancing speed, cost, and the required level of accuracy. There is no single "best" method for all situations.
Accuracy vs. Speed
Directly analyzing a solid piece is very fast but risks inaccuracy if the surface isn't perfectly prepared or if the material is inhomogeneous.
Creating fused beads provides the highest accuracy and precision by eliminating physical effects, but it is destructive, slow, and requires specialized equipment. Pressed pellets offer a reliable compromise between the two.
Sample Integrity and Contamination
Grinding and pressing a sample can introduce contamination from the grinder or binder material. This is a critical concern when analyzing for trace elements.
Likewise, the flux used in fused beads dilutes the sample. This can make it difficult to detect elements present at very low concentrations (parts per million).
Destructive vs. Non-destructive
Placing a finished part or unique artifact directly in the analyzer can be completely non-destructive. However, cutting, grinding, or fusing a sample permanently alters or destroys it. You must consider if the sample can be sacrificed for the analysis.
Making the Right Choice for Your Goal
Base your sample preparation strategy on your analytical needs and the nature of your sample.
- If your primary focus is rapid QC or material identification: Direct analysis of a solid with a clean, flat surface is often sufficient.
- If your primary focus is high-accuracy quantitative analysis of powders: Creating pressed pellets is the industry standard, offering a great balance of accuracy and effort.
- If your primary focus is certifiable, high-precision results for complex materials: Fused beads are the superior choice, as they remove nearly all sources of physical error.
- If your sample is precious or cannot be destroyed: You must use non-destructive direct analysis and acknowledge the potential for inaccuracies due to surface texture or inhomogeneity.
Ultimately, proper sample preparation is the foundation upon which reliable XRF data is built.
Summary Table:
| Preparation Method | Best For | Key Consideration |
|---|---|---|
| Direct Solid Analysis | Rapid QC, material ID | Surface must be flat and clean |
| Pressed Pellets | Powders, soils, cements | Balances accuracy and effort |
| Fused Beads | High-precision, complex materials | Eliminates physical errors |
Achieve precise and reliable XRF analysis with KINTEK.
Proper sample preparation is the cornerstone of accurate results. Whether you are analyzing metals, powders, or unique materials, the right equipment and consumables are critical. KINTEK specializes in high-quality lab equipment, including pellet presses, fusion furnaces, and grinding mills, designed to help you create the perfect sample for your XRF analyzer.
Don't let improper preparation compromise your data. Contact our experts today to discuss your specific application and find the ideal solution for your laboratory needs.
Get in touch via our Contact Form to learn more!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Three-dimensional electromagnetic sieving instrument
- Lab Internal Rubber Mixer Rubber Kneader Machine for Mixing and Kneading
People Also Ask
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- How can corrosion of the sample holder be prevented when using corrosive chemicals? Protect Your Lab's Integrity
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions
- What is the difference between XRF and XRD techniques? A Guide to Choosing the Right Analytical Tool



















