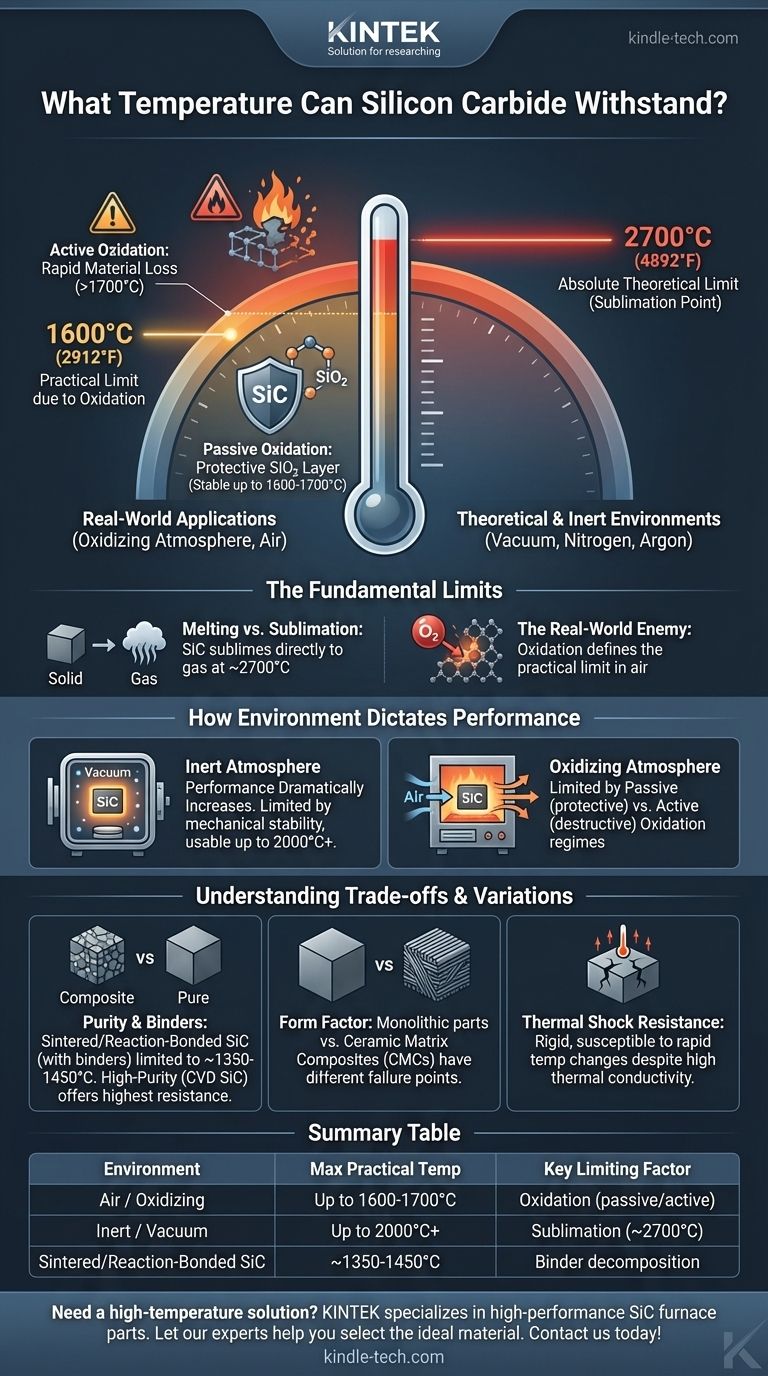
In most real-world applications, silicon carbide (SiC) can withstand continuous operating temperatures up to approximately 1600°C (2912°F) in an oxidizing atmosphere like air. While its theoretical limit is much higher, its practical performance is dictated almost entirely by its surrounding environment and its specific grade or form.
The question isn't simply "how hot can SiC get," but rather "at what temperature does SiC begin to degrade in a specific environment?" The true limiting factor for most applications is not melting, but oxidation, which begins to compromise the material long before it reaches its sublimation point.

The Fundamental Limits of Silicon Carbide
To properly use silicon carbide, you must understand the difference between its absolute thermal limit and its practical operational ceiling. These are two very different numbers driven by different physical phenomena.
Melting vs. Sublimation
Unlike many metals that have a clear melting point, silicon carbide does not melt at atmospheric pressure. Instead, it sublimates, turning directly from a solid into a gas.
This sublimation occurs at an extremely high temperature, around 2700°C (4892°F). This represents the absolute theoretical temperature limit of the material itself, but this is only achievable in a vacuum or a fully inert atmosphere.
The Real-World Enemy: Oxidation
For any application exposed to air or oxygen, the practical temperature limit is defined by oxidation. Fortunately, SiC has a unique defense mechanism.
As it heats up in the presence of oxygen, it forms a thin, stable layer of silicon dioxide (SiO₂) on its surface. This process, known as passive oxidation, creates a protective barrier that prevents further, rapid degradation of the underlying SiC.
This passive oxide layer is highly effective up to about 1600-1700°C (2912-3092°F), depending on the purity of the SiC. This range is the realistic maximum operating temperature for long-term, stable use in air.
How Environment Dictates Performance
The atmosphere in which SiC operates is the single most important factor in determining its maximum service temperature.
In an Inert Atmosphere (e.g., Argon, Nitrogen)
When oxygen is removed from the equation, silicon carbide's performance dramatically increases. In inert or vacuum environments, it is no longer limited by oxidation.
Here, the limiting factor becomes its mechanical stability. SiC can be used reliably up to 2000°C (3632°F) or even higher, approaching its sublimation point. This makes it a premier material for high-temperature furnace components and semiconductor manufacturing equipment.
The Onset of Active Oxidation
Above approximately 1700°C in an oxidizing atmosphere, the protective mechanism fails. The stable SiO₂ layer can no longer form properly.
Instead, the silicon carbide reacts with oxygen to form silicon monoxide (SiO) gas. This active oxidation process rapidly consumes the material, leading to catastrophic failure. Operating SiC in this regime is unsustainable.
Understanding the Trade-offs and Variations
Not all silicon carbide is created equal. The manufacturing method and final form introduce trade-offs that directly impact temperature resistance and overall performance.
The Role of Purity and Binders
Most commercial SiC parts are not pure SiC. They are made by sintering SiC powder with binding agents to form a dense, solid object. These binders often have a lower melting or decomposition temperature than the SiC itself.
Sintered SiC or reaction-bonded SiC may have a lower maximum use temperature, sometimes limited to 1350-1450°C (2462-2642°F), because the binding phase becomes the weak link. In contrast, high-purity materials like CVD SiC (made by chemical vapor deposition) contain no binders and offer the highest temperature resistance.
Form Factor: Monolithic vs. Composites
The shape and structure of the final part matter. A solid, monolithic SiC component, like a seal or a nozzle, will behave as described above.
However, SiC is also used as reinforcing fibers in Ceramic Matrix Composites (CMCs) for aerospace applications. In a CMC, the failure might not be the SiC fiber itself but the interface between the fiber and the matrix material, which could have a lower temperature limit.
Thermal Shock Resistance
While SiC has excellent high-temperature strength, its rigidity makes it susceptible to thermal shock—failure from rapid temperature changes. Its high thermal conductivity helps mitigate this risk by distributing heat quickly, but extreme temperature gradients can still induce cracks.
Making the Right Choice for Your Application
Selecting the correct grade and anticipating the operating environment are critical for success.
- If your primary focus is extreme heat in a controlled, inert atmosphere: Use high-purity, binderless SiC (like CVD SiC) to safely operate in the 1700-2200°C range.
- If your primary focus is long-term stability in air: Design around a maximum continuous temperature of 1600°C to leverage SiC's protective passive oxidation layer.
- If your primary focus is cost-effectiveness for moderate-to-high temperatures: A reaction-bonded or sintered SiC is a practical choice, but respect its lower operating ceiling, typically around 1400°C.
Understanding these critical distinctions is the key to successfully harnessing the remarkable thermal capabilities of silicon carbide.
Summary Table:
| Environment | Max Practical Temperature | Key Limiting Factor |
|---|---|---|
| Air / Oxidizing Atmosphere | Up to 1600-1700°C | Oxidation (passive/active) |
| Inert Atmosphere / Vacuum | Up to 2000°C+ | Sublimation (~2700°C) |
| Sintered/Reaction-Bonded SiC | ~1350-1450°C | Binder decomposition |
Need a high-temperature solution for your lab? The right silicon carbide component can dramatically improve your process efficiency and durability. KINTEK specializes in lab equipment and consumables, including high-performance SiC furnace parts designed for stability in both oxidizing and inert environments. Let our experts help you select the ideal material for your specific temperature and atmospheric requirements. Contact us today to discuss your application!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Optical Window Glass Substrate Wafer Quartz Plate JGS1 JGS2 JGS3
- Float Soda-Lime Optical Glass for Laboratory Use
- MgF2 Magnesium Fluoride Crystal Substrate Window for Optical Applications
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
People Also Ask
- What is porcelain powder used for? From Dental Crowns to Fine China
- What are the thermal properties of silicon carbide nanoparticles? Unlock Superior Heat Management
- What is the thermal stability of SiC? Withstand Extreme Heat Up to 2700°C
- Does silicon carbide absorb water? Discover Its Inherent Moisture Resistance for Demanding Applications
- How do ceramic insulators contribute to SHS experimental safety? Enhance Your Lab’s Ignition Precision and Protection
- Why is density important in ceramics? It's the Key to Mechanical Strength and Performance
- What is meant by ceramic powder? The Engineered Blueprint for Advanced Ceramics
- What are the three types of dental ceramic? A Guide to Balancing Aesthetics & Strength