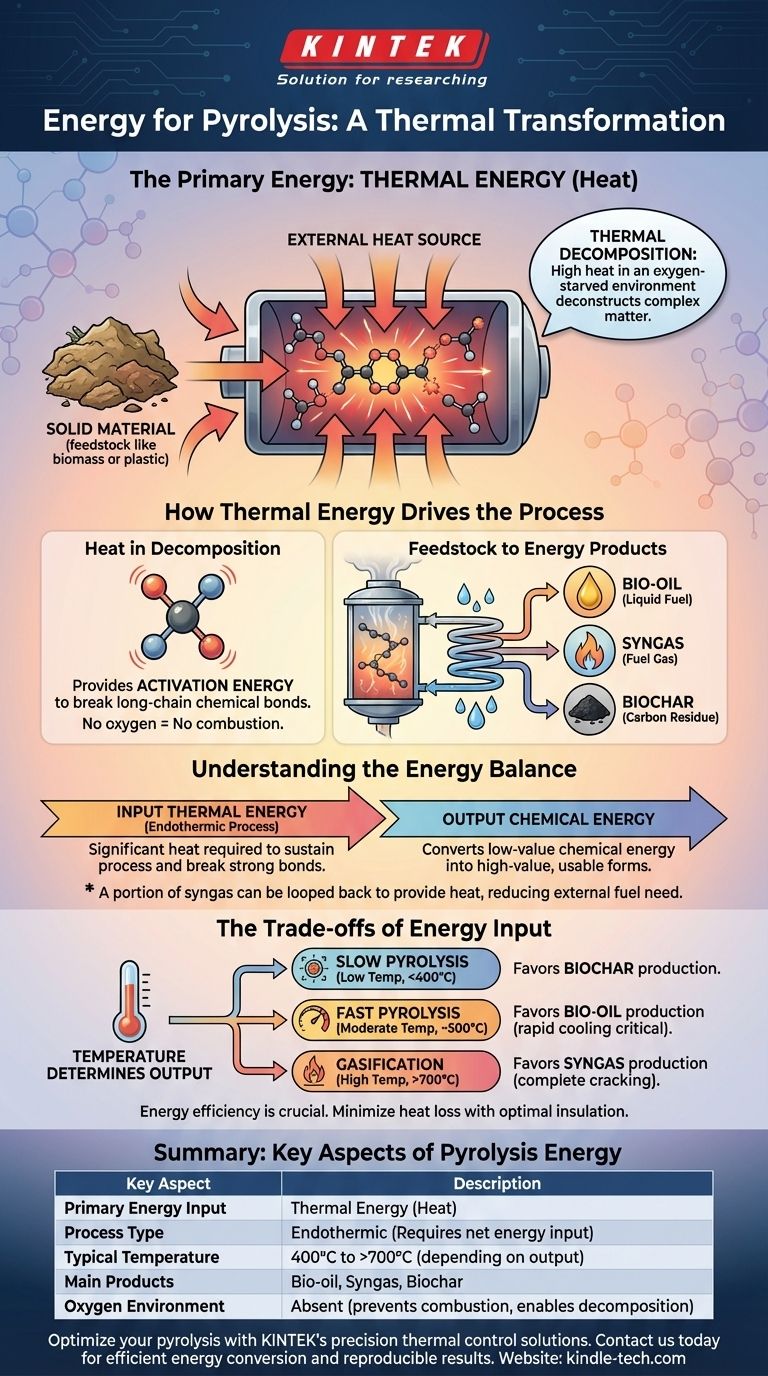
The primary energy required to break down material during pyrolysis is thermal energy. This process, known as thermal decomposition, uses high heat in an oxygen-starved environment to break down complex organic matter. Instead of burning the material, the heat systematically deconstructs its chemical bonds, transforming it into new, energy-rich products.
Pyrolysis is best understood as an energy transformation process. It consumes an initial input of thermal energy (heat) to convert the latent chemical energy stored in a solid feedstock into more accessible and valuable forms of chemical energy: syngas, bio-oil, and biochar.
How Thermal Energy Drives the Process
The Role of Heat in Decomposition
The core function of the energy input is to provide the activation energy necessary to break down long-chain polymer molecules found in materials like biomass, plastics, or tires. This heat causes the molecules to vibrate violently until their chemical bonds rupture.
This decomposition happens in the absence of oxygen. If oxygen were present, the material would simply combust (burn), releasing its energy inefficiently as light and heat. Pyrolysis avoids combustion to capture the energy in new chemical forms.
From Solid Feedstock to Energy Products
As the feedstock is heated, it breaks down into smaller, volatile molecules. These molecules are released as vapors.
Upon cooling and condensing, these vapors form a liquid known as bio-oil or pyrolysis oil, which contains a high concentration of chemical energy. The non-condensable gases form syngas, a fuel gas mixture. The solid residue left behind is a carbon-rich substance called biochar.
Understanding the Energy Balance: Input vs. Output
Endothermic by Nature
The overall pyrolysis process is endothermic, meaning it requires a net input of energy to sustain itself. The initial heating and the breaking of strong chemical bonds consume significant thermal energy.
While some secondary reactions within the pyrolysis process can be exothermic (releasing heat), this is not enough to power the entire operation. A continuous external heat source is almost always required.
A Conversion, Not a Loss
The input thermal energy is not lost; it is used to upgrade the energy in the feedstock. It converts the stable, low-value chemical energy of a solid waste product into the high-value, readily usable chemical energy of liquid and gas fuels.
In many industrial systems, a portion of the syngas produced is looped back and burned to provide the heat needed for the process. This creates a partially self-sustaining system, reducing the need for external fuel.
The Trade-offs of Energy Input
The Need for an External Heat Source
The primary operational challenge and cost of pyrolysis is generating and applying the high temperatures required, which typically range from 400°C to over 700°C. This energy cost is a critical factor in the economic viability of a pyrolysis plant.
Temperature Determines the Output
The specific temperature and heating rate directly control the final product yields. There is no single "best" temperature; it is a trade-off based on the desired outcome.
- Slow Pyrolysis (Low Temp): Favors biochar production.
- Fast Pyrolysis (Moderate Temp, ~500°C): Favors bio-oil production.
- Gasification (High Temp, >700°C): Favors syngas production.
Inevitable Energy Losses
As with any thermal process, energy efficiency is a key concern. Heat will inevitably be lost to the reactor walls and the surrounding environment. Optimizing insulation and heat recovery is crucial for making the process economically and environmentally sound.
How to Apply This to Your Goal
The way you control the input thermal energy depends entirely on your desired output.
- If your primary focus is maximizing liquid fuel (bio-oil): You need a fast pyrolysis process with precise temperature control around 500°C and very rapid cooling of the vapors.
- If your primary focus is creating biochar for agriculture: You should use a slower pyrolysis process at lower temperatures (350-500°C) to maximize the solid residue yield.
- If your primary focus is generating syngas for electricity: You must operate at much higher temperatures (above 700°C) to ensure the complete thermal cracking of the feedstock into gaseous components.
Understanding that pyrolysis is fundamentally a thermal energy conversion process is the key to optimizing it for any application.

Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Energy Input | Thermal Energy (Heat) |
| Process Type | Endothermic (requires net energy input) |
| Typical Temperature | 400°C to >700°C, depending on desired output |
| Main Products | Bio-oil, Syngas, Biochar |
| Oxygen Environment | Absent (prevents combustion, enabling chemical decomposition) |
Ready to optimize your pyrolysis process with precision thermal control? At KINTEK, we specialize in high-performance lab equipment and consumables tailored for pyrolysis applications. Whether you're researching bio-oil yields, biochar production, or syngas generation, our reactors and heating systems ensure efficient energy conversion and reproducible results. Contact us today to explore how our solutions can advance your laboratory's pyrolysis projects and maximize your output efficiency!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Vacuum Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
People Also Ask
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- How does a high-pressure reactor demonstrate its value in accelerated aging? Predict Catalyst Durability Fast


















