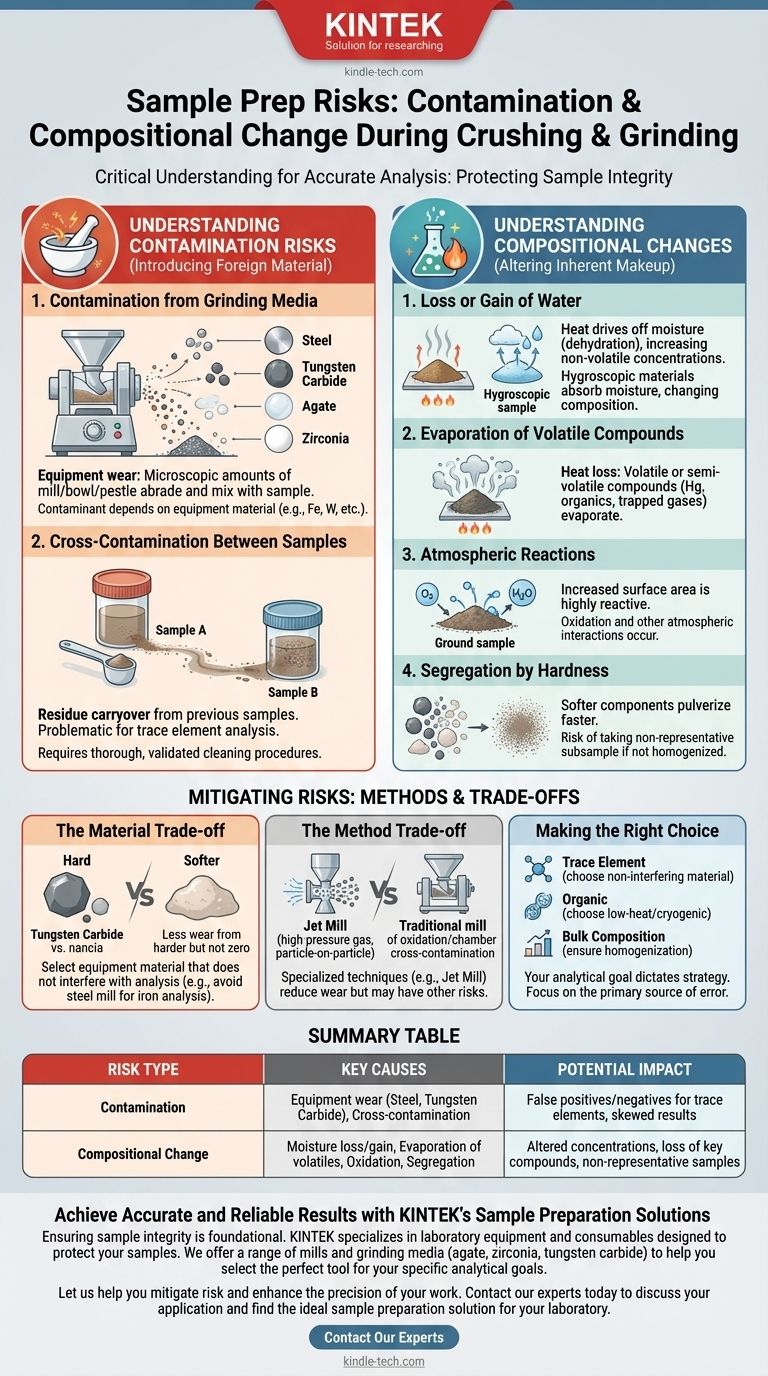
When preparing a sample for analysis, it is critical to understand that crushing and grinding are not neutral acts. These processes can introduce two major types of errors: contamination from external sources and fundamental changes to the sample's own composition. Contamination stems from equipment wear and residue from previous samples, while compositional changes are driven by factors like moisture loss, oxidation, and the evaporation of volatile compounds.
The physical act of crushing and grinding is an active process that introduces significant risks to sample integrity. The goal is not merely to reduce particle size, but to do so while consciously mitigating two key threats: the introduction of foreign materials (contamination) and the alteration of the sample's inherent chemical makeup (compositional change).

Understanding Contamination Risks
Contamination involves introducing foreign material into your sample. This foreign material can mask or mimic the components you intend to measure, leading to inaccurate results.
Contamination from Grinding Media
The most common source of contamination is the grinding equipment itself. The immense force and friction involved in crushing and grinding cause microscopic amounts of the mill, bowl, or mortar and pestle to abrade and mix with your sample.
The specific contaminant depends entirely on the material of your equipment (e.g., steel, tungsten carbide, agate, zirconia).
Cross-Contamination Between Samples
A second risk is cross-contamination, where residue from a previously processed sample is carried over into the current one. This is especially problematic when analyzing for trace elements, where even a tiny amount of carryover can dramatically skew results.
Thorough and validated cleaning procedures between samples are essential to prevent this.
Understanding Compositional Changes
Even if no foreign material is introduced, the grinding process can alter the inherent chemical and physical nature of the sample itself.
Loss or Gain of Water
The heat generated by friction during grinding can drive off moisture, a process known as dehydration. This artificially increases the concentration of all other non-volatile components in the sample.
Conversely, a hygroscopic (water-attracting) material can absorb moisture from the atmosphere, especially once its surface area is increased by grinding.
Evaporation of Volatile Compounds
Just as water can be driven off, other volatile or semi-volatile compounds can be lost due to heat. This is a significant concern when analyzing for substances like mercury, certain organics, or gases trapped within the material.
Atmospheric Reactions
Grinding dramatically increases the sample's surface area. This new, unpassivated surface is highly reactive and can readily interact with the atmosphere, most commonly leading to oxidation (reaction with oxygen).
Segregation by Hardness
Many samples are composites of materials with different hardness levels. During grinding, softer components pulverize more easily than harder ones. If the sample is not perfectly homogenized after grinding, you risk taking a subsample that is not representative of the original bulk material.
Mitigating Risks: Methods and Trade-offs
There is no single "perfect" grinding method; each involves trade-offs designed to minimize specific types of errors.
The Material Trade-off
Choosing a harder grinding material (like tungsten carbide) reduces the amount of contamination from wear, but it doesn't eliminate it. The key is to select equipment made from a material that does not interfere with your subsequent analysis.
For example, you would not use a steel mill if you were analyzing for iron or chromium.
The Method Trade-off
Specialized techniques exist to target specific problems. A jet mill, for instance, uses high-pressure gas to force particles to collide with each other.
This particle-on-particle grinding dramatically reduces contamination from equipment wear. However, it does not eliminate the risk of compositional changes like oxidation or cross-contamination from the mill chamber.
Making the Right Choice for Your Goal
Your analytical goal must dictate your sample preparation strategy. Consider the primary source of error you need to avoid.
- If your primary focus is trace element analysis: Your main concern is contamination from grinding media. Choose equipment made from a material you are not analyzing for.
- If your primary focus is preserving organic compounds: Your main concern is heat-induced loss of volatiles. Consider methods that generate less heat or cryogenic (freeze) grinding.
- If your primary focus is bulk composition: Your main concern is representativeness. Ensure your final ground sample is thoroughly homogenized to counteract segregation by hardness.
Ultimately, a successful analysis depends on a sample preparation strategy that actively preserves the material's original state.
Summary Table:
| Risk Type | Key Causes | Potential Impact on Analysis |
|---|---|---|
| Contamination | Equipment wear (steel, tungsten carbide), cross-contamination from previous samples | False positives/negatives for trace elements, skewed results |
| Compositional Change | Moisture loss/gain, evaporation of volatiles, oxidation, segregation by hardness | Altered concentrations, loss of key compounds, non-representative samples |
Achieve Accurate and Reliable Results with KINTEK's Sample Preparation Solutions
Ensuring sample integrity is the foundation of any successful analysis. The risks of contamination and compositional change during crushing and grinding are real, but they are manageable with the right equipment and expertise.
KINTEK specializes in providing laboratory equipment and consumables designed to protect your samples. We offer a range of mills and grinding media in various materials (e.g., agate, zirconia, tungsten carbide) to help you select the perfect tool for your specific analytical goals, whether you're focused on trace element analysis, organic compound preservation, or bulk composition.
Let us help you mitigate risk and enhance the precision of your work.
Contact our experts today to discuss your application and find the ideal sample preparation solution for your laboratory.
Visual Guide
Related Products
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Powerful Plastic Crusher Machine
- Mini Planetary Ball Mill Machine for Laboratory Milling
- High Energy Planetary Ball Mill Machine for Laboratory Horizontal Tank Type
- Twin Screw Extruder Plastic Granulation Machine
People Also Ask
- What is the role of an agate mortar and pestle in cathode preparation? Key Steps for Sodium-Sulfur Battery Success
- How does the size of zirconia grinding balls influence Na3PS4 synthesis? Optimize Your Electrolyte Production Speed
- Why are zirconia grinding balls of different diameters used for carbon nitride? Optimize Your Nano-Material Synthesis
- How much power does a ball mill use? A Guide to Estimating and Controlling Energy Costs
- What is the function of using an agate mortar during the precursor mixing stage of sulfide solid electrolyte synthesis?
- What is the role of a mechanical rotating ball mill in Li-C anode preparation? Optimize Surface Coating & Conductivity
- What is a ball mill used for in ceramics? Achieve Ultimate Control Over Glaze and Clay Quality
- What are the advantages of a colloid mill? Achieve Superior Particle Size Reduction and Stable Emulsions



















