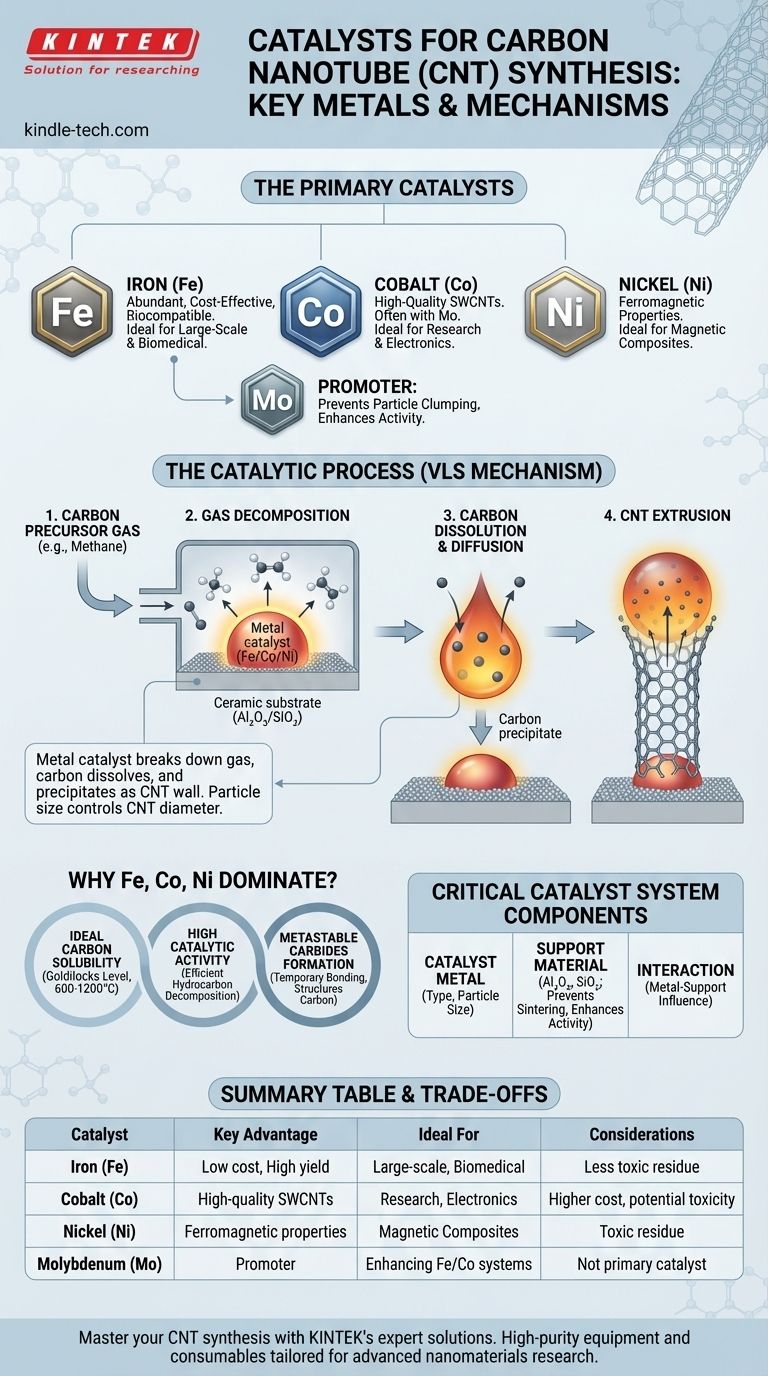
The primary metals used as catalysts for synthesizing carbon nanotubes (CNTs) are a specific group of transition metals. The most common and effective catalysts are Iron (Fe), Cobalt (Co), and Nickel (Ni). These metals, or their alloys, are essential for breaking down carbon-containing precursor gases and assembling the carbon atoms into the unique hexagonal lattice of a nanotube.
The choice of a catalyst for CNT synthesis is not merely about selecting a metal, but about engineering a system. The effectiveness of Iron, Cobalt, and Nickel lies in their unique ability to form metastable carbides and facilitate carbon diffusion at high temperatures, with the final CNT properties being heavily influenced by the catalyst's particle size and its interaction with a support material.

The Fundamental Role of the Catalyst
The Catalyst's Core Function
A catalyst's job in CNT synthesis is twofold. First, it must efficiently break down the bonds of a carbon-containing gas (like methane, ethylene, or acetylene). Second, it must provide a template surface upon which the carbon atoms can reassemble into the graphitic structure of a nanotube.
The Growth Mechanism
The process is most often described by the Vapor-Liquid-Solid (VLS) or Vapor-Solid-Solid (VSS) mechanism. The metal catalyst forms a nanoparticle (either liquid or solid at synthesis temperatures) which acts as a seed.
Carbon from the precursor gas dissolves into this nanoparticle. Once the nanoparticle becomes supersaturated with carbon, the carbon begins to precipitate out, forming the cylindrical wall of the nanotube. The catalyst particle essentially functions as a nanoscale "print head" extruding the CNT.
Why Iron, Cobalt, and Nickel Dominate
Ideal Carbon Solubility
The key to Fe, Co, and Ni is their "Goldilocks" level of carbon solubility at typical synthesis temperatures (600-1200°C). Their solubility is high enough to facilitate the process but low enough that the carbon precipitates out easily to form the tube.
Metals with too low a solubility won't work, and metals that form extremely stable carbides (like titanium) will "poison" the catalyst by locking up the carbon permanently.
Catalytic Activity
These three metals show high catalytic activity for decomposing hydrocarbon gases into the elemental carbon needed for growth. This efficiency is crucial for achieving a high yield of CNTs.
Formation of Metastable Carbides
Fe, Co, and Ni form intermediate, unstable compounds with carbon (metastable carbides). This temporary bonding is critical for holding onto the carbon long enough to structure it before it precipitates as the nanotube wall.
The Role of Molybdenum (Mo)
While not a primary growth catalyst on its own, Molybdenum (Mo) is frequently used as a co-catalyst or "promoter," especially with Iron or Cobalt. It helps to keep the catalyst particles small and well-dispersed at high temperatures, which is vital for growing high-quality, small-diameter CNTs.
The Critical Importance of the Support Material
The catalyst metal is almost never used in bulk form. Instead, it is deposited as nanoparticles onto a ceramic support material.
Controlling Catalyst Particle Size
The diameter of a carbon nanotube is directly determined by the size of the catalyst nanoparticle it grows from. The support material, typically alumina (Al₂O₃) or silica (SiO₂), provides a high-surface-area substrate that prevents the tiny metal particles from clumping together (sintering) at high temperatures.
Enhancing Catalytic Activity
The interaction between the metal nanoparticle and the support can significantly enhance catalytic activity. This metal-support interaction can influence the chemical state of the catalyst and improve its efficiency in producing high-quality CNTs.
Understanding the Trade-offs
Catalyst Purity and Contamination
The single biggest drawback is that the metal catalyst remains in the final CNT product. Removing these impurities requires harsh post-processing with acids, which can damage the nanotubes themselves.
This contamination is a major problem for applications in electronics (where metals alter conductivity) and biomedicine (where Co and Ni can be toxic).
Cost and Availability
Iron (Fe) is abundant, inexpensive, and relatively non-toxic, making it the preferred catalyst for large-scale, low-cost production and for many biological applications.
Cobalt (Co) is more expensive but is often cited as the most effective catalyst for producing high-quality single-walled carbon nanotubes (SWCNTs), especially when paired with Mo.
Difficulty in Controlling Structure
While these catalysts are effective at producing CNTs, it remains exceptionally difficult to control the exact structure (chirality) of the nanotube being grown. For most synthesis methods, the result is a mix of different types of nanotubes, which limits their use in highly specific electronic applications.
Making the Right Choice for Your Goal
The ideal catalyst depends entirely on the desired outcome of the synthesis.
- If your primary focus is high yield and low cost: Iron (Fe) is the industry standard due to its low price, high activity, and relative safety.
- If your primary focus is high-quality SWCNTs: Cobalt (Co), often combined with a Molybdenum (Mo) promoter, is a well-established choice for research-grade material.
- If your primary focus is biocompatibility: Iron (Fe) is the superior choice, as residual iron particles are far less toxic to biological systems than cobalt or nickel.
- If your primary focus is magnetic CNT composites: Nickel (Ni) is often explored due to its inherent ferromagnetic properties, which can be imparted to the final material.
Ultimately, mastering CNT synthesis is about controlling the catalyst system—the metal, its size, and its support—to build the precise nanostructures your application demands.
Summary Table:
| Catalyst Metal | Key Advantage | Ideal For |
|---|---|---|
| Iron (Fe) | Low cost, high yield, biocompatible | Large-scale production, biomedical applications |
| Cobalt (Co) | High-quality SWCNTs, effective with Mo promoter | Research-grade materials, electronics |
| Nickel (Ni) | Ferromagnetic properties | Magnetic CNT composites |
| Molybdenum (Mo) | Promoter, prevents particle clumping | Enhancing Fe/Co catalyst systems |
Master your CNT synthesis with KINTEK's expert solutions. Selecting the right catalyst is critical for achieving the desired quality and properties of your carbon nanotubes. KINTEK specializes in providing high-purity lab equipment and consumables, including catalyst materials and support systems, tailored for advanced nanomaterials research. Let our expertise help you optimize your catalyst system for superior results. Contact our specialists today to discuss your specific CNT synthesis needs and discover how we can support your laboratory's success.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- CVD Diamond Cutting Tool Blanks for Precision Machining
- CVD Diamond Domes for Industrial and Scientific Applications
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What is the meaning of deposition of vapor? A Guide to Thin-Film Coating Technologies
- What is the primary function of a CVD system in LDIP preparation? Engineering Superhydrophobic Micro-Nano Structures
- What is chemical vapour deposition for thin films? A Guide to High-Performance Surface Engineering
- What is the process of LPCVD? Master High-Purity, Uniform Thin-Film Deposition
- How does Chemical Vapor Deposition (CVD) equipment improve the lithiophilicity of copper? Boost Battery Stability
- What is the fundamentals of chemical vapour deposition? A Guide to High-Performance Thin Films
- Why different coatings are applied on carbide tool inserts? Boost Performance, Wear, and Heat Resistance
- How are thin film nanoparticles prepared? A Guide to PVD and CVD Deposition Methods



















