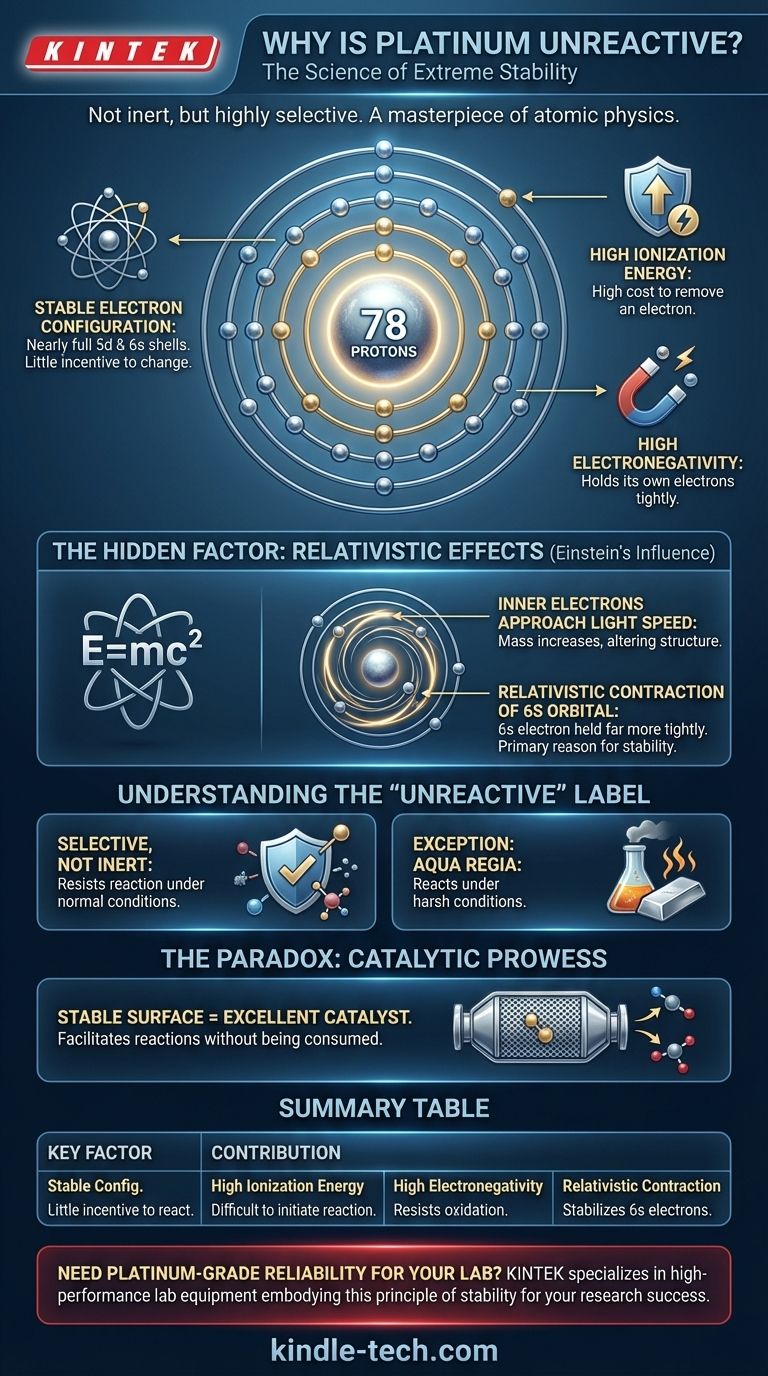
At its core, platinum's unreactivity is not a matter of simplicity, but of immense atomic complexity. It is profoundly stable because its massive nucleus creates powerful relativistic effects that fundamentally alter its electron structure, making its electrons exceptionally difficult to remove or share in a chemical reaction. This is combined with a high ionization energy and a filled set of inner electron shells, creating a uniquely steadfast element.
The term "unreactive" is misleading; platinum is not inert, but highly selective. Its stability arises from a combination of its electron configuration and powerful relativistic effects, a consequence of its heavy atomic nucleus that makes it energetically unfavorable to participate in most chemical reactions.

The Atomic Blueprint for Stability
To understand platinum's resilience, we must look at its atomic structure. Several factors work in concert to create its remarkable chemical composure.
A Full and Stable Electron Configuration
Platinum possesses a dense core of electrons in stable, filled shells. Its outermost, or valence, electrons reside in the 5d and 6s orbitals.
These orbitals are nearly full, a configuration that is energetically stable. Atoms tend to react to achieve a more stable state, but platinum is already very close to one, giving it little "incentive" to change.
High Ionization Energy: The High Cost of Reaction
Ionization energy is the energy required to remove an electron from an atom. For a chemical reaction to occur, electrons must typically be shared or transferred.
Platinum has a very high first ionization energy. A great deal of energy is needed to pull even one electron away, making the formation of a positive platinum ion an energetically costly and unfavorable process.
High Electronegativity for a Metal
Electronegativity is a measure of an atom's ability to attract and hold onto electrons. Platinum's electronegativity is high for a metal, meaning it holds its own electrons very tightly.
This reluctance to give up electrons is a primary reason it resists oxidation, the process that causes metals like iron to rust.
The Hidden Factor: Relativistic Effects
For very heavy elements like platinum, a phenomenon predicted by Einstein's theory of relativity becomes a dominant chemical force. This is the true expert insight into its stability.
When Electrons Approach the Speed of Light
Platinum has a massive, positively charged nucleus (78 protons). To avoid spiraling into this nucleus, the innermost electrons must orbit at a significant fraction of the speed of light.
According to relativity, as an object's speed approaches the speed of light, its mass increases. This happens to platinum's inner electrons, which in turn affects the entire atomic structure.
The Contraction of the 6s Orbital
The heavier, faster inner electrons pull the outer 6s electron orbital closer to the nucleus, a process called relativistic contraction.
This contracted 6s orbital is more stable and its electron is held far more tightly than periodic trends would predict. This effect is a primary contributor to platinum's high ionization energy and is a key reason for its lack of reactivity. It is also the same effect that gives gold its characteristic yellow color.
Understanding the "Unreactive" Label
The term "unreactive" is a simplification. It is more accurate to describe platinum as being chemically selective, resisting reaction under normal conditions but participating under specific, extreme ones.
Not Inert, Just Selective
Unlike a truly inert noble gas, platinum can and does react. However, the conditions required are often harsh, involving high temperatures or extremely corrosive agents.
The Power of Aqua Regia
The classic example is platinum's reaction with aqua regia, a highly corrosive mixture of nitric acid and hydrochloric acid. This potent mixture is one of the few chemical agents that can dissolve platinum at room temperature, demonstrating that its stability can be overcome.
Catalytic Prowess: The Other Side of Stability
Paradoxically, platinum's stability is what makes it an excellent catalyst. Its surface provides a stable, non-reactive platform on which other chemical reactions can occur more efficiently.
Because the platinum atoms do not readily bond with the reactants, they can facilitate a reaction without being consumed in the process. This is why it is essential in catalytic converters, where it helps convert toxic pollutants into less harmful substances.
Making the Right Choice for Your Goal
Understanding platinum's stability is key to leveraging its properties in science and industry.
- If your primary focus is material selection for a harsh environment: Platinum's resistance to corrosion and oxidation makes it the premier choice for long-lasting jewelry, medical implants, and laboratory electrodes.
- If your primary focus is understanding chemical catalysis: Platinum's surface stability is the reason it can facilitate reactions without being consumed, making it a model for developing efficient industrial and environmental catalysts.
- If your primary focus is predicting chemical properties: Platinum is a prime example of how, for heavy elements, you must consider relativistic effects, which can dramatically alter chemical behavior and override simpler periodic trends.
Platinum's chemical quietness is not a passive trait, but an active consequence of the extreme physics governing its massive atomic core.
Summary Table:
| Key Factor | Contribution to Platinum's Stability |
|---|---|
| Stable Electron Configuration | Nearly full 5d and 6s valence shells provide little incentive to react. |
| High Ionization Energy | Requires a large amount of energy to remove an electron, making reaction initiation difficult. |
| High Electronegativity | Holds onto its own electrons tightly, resisting oxidation. |
| Relativistic Contraction | Inner electrons moving near light speed contract the 6s orbital, stabilizing its electrons beyond normal trends. |
Need Platinum-Grade Reliability for Your Lab?
Understanding the profound stability of elements like platinum is key to selecting the right materials and equipment for demanding applications. At KINTEK, we specialize in providing high-performance lab equipment and consumables that embody this same principle of reliability.
Whether you require corrosion-resistant apparatus for harsh chemical environments or catalytic systems for your research, our solutions are designed for precision and longevity.
Let KINTEK be the stable foundation for your laboratory's success. Contact our experts today to discuss how we can meet your specific needs.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- High-Purity Titanium Foil and Sheet for Industrial Applications
- High Purity Gold Platinum Copper Iron Metal Sheets
People Also Ask
- What is pyrolysis oil worth? A Guide to Valuing This Complex Biofuel
- Is deposition physical or chemical? Unraveling the Science of Phase Transitions
- What is pressing and sintering of metals? A Guide to High-Strength Metal Parts Manufacturing
- What is the function of a laboratory magnetic stirrer in photocatalytic degradation? Achieve Kinetic Accuracy
- What are the fundamentals of sputtering? Master the Art of High-Quality Thin Film Deposition
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- What are the advantages of electron beam hardening? Achieve Superior Precision and Speed
- How do you calculate the capacity of a filter press? Use Pilot Testing for Accurate Sizing









