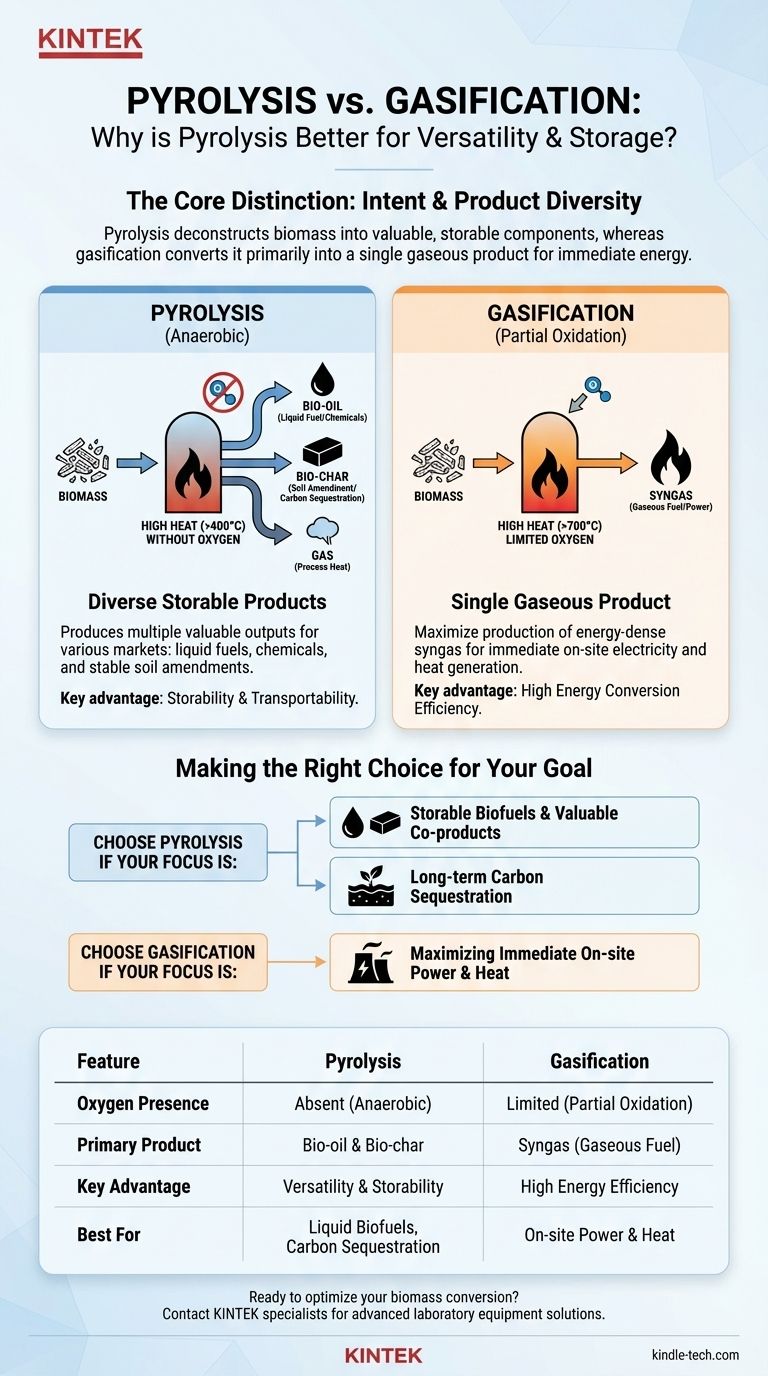
Pyrolysis is considered superior to gasification when the primary goal is to create a diverse range of valuable and storable products, such as liquid bio-oil and solid bio-char. While gasification is a powerful process, its focus is narrower, excelling primarily at generating a gaseous fuel for immediate power and heat production.
The core distinction is one of intent. Pyrolysis deconstructs biomass into valuable, storable components (liquid, solid, and gas), whereas gasification converts biomass almost entirely into a single gaseous product (syngas) for immediate energy generation.

The Fundamental Difference: The Role of Oxygen
The choice between pyrolysis and gasification hinges on one critical element: the presence or absence of oxygen during the process. This single factor dictates the chemical reactions that occur and, consequently, the final products.
Pyrolysis: Thermal Decomposition Without Oxygen
Pyrolysis is the thermal decomposition of organic material at high temperatures in a completely oxygen-free environment.
Without oxygen, the material cannot burn. Instead, the heat breaks down complex organic polymers into simpler, smaller molecules, which are then captured as distinct products.
This process essentially "un-builds" the biomass, separating it into a liquid fraction (bio-oil), a solid fraction (bio-char), and a gaseous fraction (syngas).
Gasification: Partial Oxidation for Gaseous Fuel
Gasification exposes biomass to very high temperatures (>700°C) with a controlled, limited amount of oxygen present.
This small amount of oxygen is not enough for full combustion. Instead, it drives a series of chemical reactions that convert the vast majority of the biomass into synthesis gas (syngas)—a mixture primarily composed of hydrogen (H₂) and carbon monoxide (CO).
You are essentially sacrificing a small portion of the biomass to oxidation to provide the energy needed to convert the rest into a high-energy gas.
A Head-to-Head Product Comparison
The superiority of pyrolysis in certain applications becomes clear when you compare the outputs of each process.
Pyrolysis Yields: Bio-oil and Bio-char
The unique advantage of pyrolysis is its ability to produce multiple valuable outputs.
Bio-oil, often called pyrolysis oil, is a liquid that can be stored, transported, and upgraded into renewable transportation fuels or specialty chemicals. This makes it a direct, storable substitute for some fossil fuel applications.
Bio-char is a stable, carbon-rich solid similar to charcoal. It is highly valued as a soil amendment that improves water retention and soil fertility while sequestering carbon for hundreds of years.
Gasification Yields: Syngas
Gasification is designed to maximize the production of one primary product: syngas.
Syngas is an energy-dense fuel, but it is a gas. It is difficult and expensive to store or transport over long distances. Its main application is for immediate use on-site in gas engines or turbines to generate electricity and heat.
Understanding the Trade-offs
Neither technology is universally superior. The "better" choice is determined by your strategic goal, which involves balancing product versatility against raw energy efficiency.
The Case for Pyrolysis: Product Versatility and Storage
Pyrolysis is the more versatile process. It creates multiple product streams that can serve different markets—liquid fuels, chemicals, and soil amendments. The ability to produce storable liquid and solid products is its key strategic advantage over gasification.
The Case for Gasification: Energy Conversion Efficiency
If the sole objective is to produce electricity or heat from biomass as efficiently as possible, gasification is generally considered more efficient. It is a more direct pathway for converting the chemical energy locked in biomass into a combustible gas for immediate power generation.
The Influence of Feedstock and Conditions
It is crucial to understand that the output of both processes is highly variable. The specific type of biomass used, along with process parameters like temperature and pressure, significantly influences the final product composition, yield, and overall efficiency.
Making the Right Choice for Your Goal
To select the appropriate technology, you must first define your primary objective. The answer lies in what you intend to produce.
- If your primary focus is creating storable liquid biofuels or valuable agricultural co-products: Pyrolysis is the superior choice because it uniquely yields transportable bio-oil and carbon-sequestering bio-char.
- If your primary focus is maximizing immediate on-site electricity or heat generation: Gasification is the more direct and efficient pathway for converting biomass into a gaseous fuel for power.
- If your primary focus is long-term carbon sequestration: Pyrolysis holds a distinct advantage through its production of bio-char, a highly stable form of carbon that can be locked in soil.
Ultimately, the decision rests not on which process is inherently better, but on which process best aligns with your strategic objective.
Summary Table:
| Feature | Pyrolysis | Gasification |
|---|---|---|
| Oxygen Presence | Absent (Anaerobic) | Limited (Partial Oxidation) |
| Primary Product | Bio-oil (Liquid) & Bio-char (Solid) | Syngas (Gaseous Fuel) |
| Key Advantage | Product Versatility & Storability | High Energy Conversion Efficiency |
| Best For | Liquid biofuels, soil amendments, carbon sequestration | On-site electricity and heat generation |
Ready to choose the right biomass conversion technology for your project?
At KINTEK, we specialize in advanced laboratory equipment for analyzing and optimizing pyrolysis and gasification processes. Whether your goal is to produce storable biofuels with pyrolysis or maximize energy efficiency with gasification, our experts can help you select the right tools to achieve your objectives.
Contact our specialists today to discuss your specific application and discover how KINTEK's solutions can enhance your research and development in renewable energy and sustainable materials.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- CVD Diamond for Thermal Management Applications
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas



















