We often think of science as a purely intellectual pursuit, a calculation of variables. But in the laboratory, science is a physical act. Nowhere is this truer than in an electrolytic cell.
An electrochemical experiment is a closed universe. You are trying to convince ions to move in a specific way to create a specific reaction. It is a delicate symphony of voltage and chemistry.
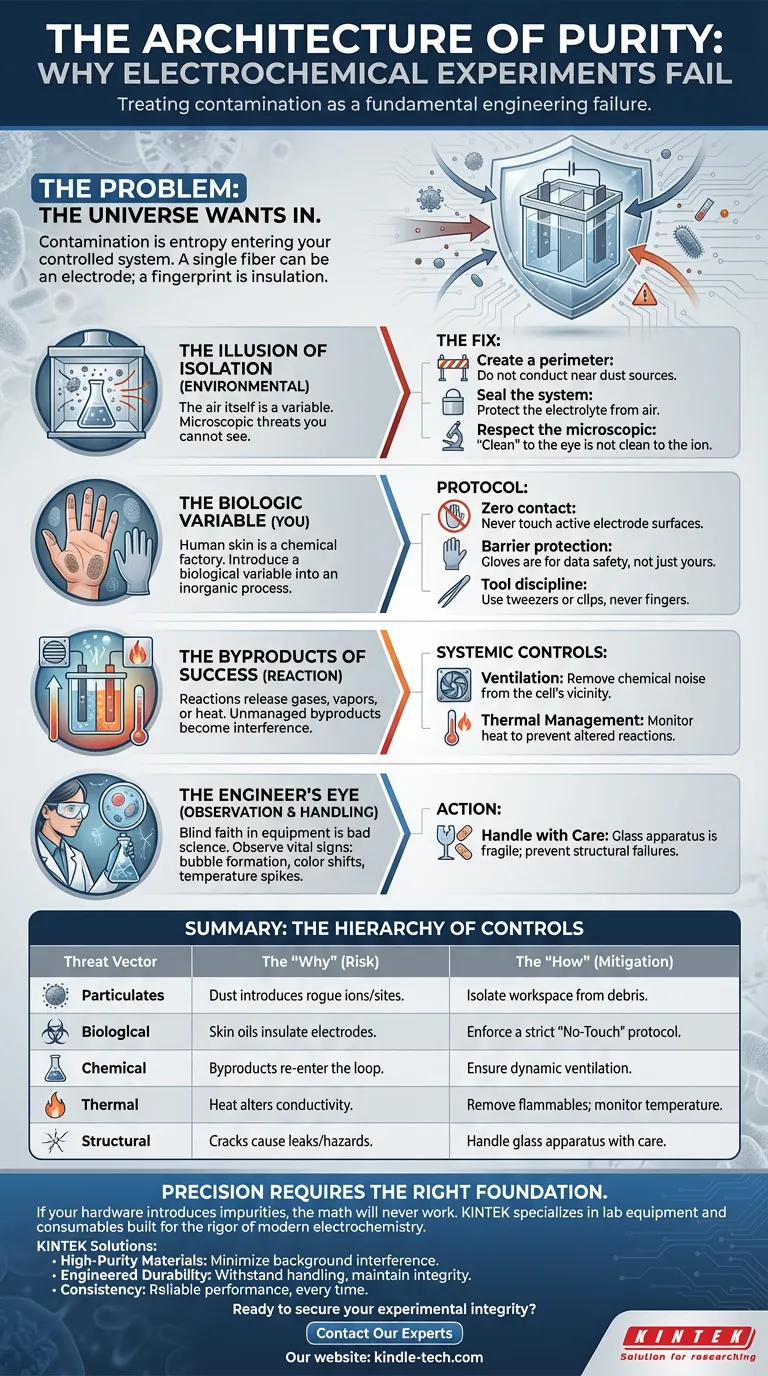
The problem is that the rest of the world wants to get in.
"Contamination" is a clinical word for a chaotic reality. It isn't just dust on a counter; it is entropy entering your controlled system. A single microscopic fiber can act as an unintended electrode. A fingerprint is a layer of organic insulation.
To achieve results that matter, we must stop treating contamination as a nuisance and start treating it as a fundamental engineering failure.
The Illusion of Isolation
The most direct threat to your data is the environment in which the data is born.
We tend to underestimate risks we cannot see. You might wipe down a bench, but if nearby operations are generating aerosols or airborne particulates, your electrolyte is already compromised. The air itself becomes a variable.
The Fix:
- Create a perimeter: Do not conduct experiments near dust-generating activities.
- Seal the system: The electrolyte is the heart of the cell. If the air touches it, the air changes it.
- Respect the microscopic: Recognizing that "clean" to the eye is not "clean" to the ion is the first step toward precision.
The Biologic Variable (You)
The most dangerous contaminant in the lab is usually the scientist.
Human skin is a chemical factory. We produce oils, salts, and residues that are fatal to electrochemical precision. Touching an electrode or the electrolyte directly does more than risk a chemical burn or electric shock—it introduces a biological variable into an inorganic process.
The best surgeons know that sterile technique is a discipline, not a chore. The same applies here.
Protocol:
- Zero contact: Never touch the active surface of electrodes.
- Barrier protection: Use gloves not just for your safety, but for the safety of your data.
- Tool discipline: Handle components with tweezers or specialized clips, never fingers.
The Byproducts of Success
Sometimes, the contamination comes from the reaction itself.
Electrolysis is transformative. It breaks bonds. This process often releases gases, vapors, or heat. If these byproducts are not managed, they turn from results into interference.
Accumulated gases can skew resistance readings. Excess heat can alter the conductivity of the electrolyte, creating a thermal runaway effect that changes the reaction rate mid-experiment.
Systemic Controls:
- Ventilation: This is not just about operator health; it is about removing chemical noise from the immediate vicinity of the cell.
- Thermal Management: Keep flammable materials away. Heat is energy, and unmanaged energy leads to explosions—the ultimate failure of experimental control.
The Engineer’s Eye
Blind faith in equipment is a recipe for bad science.
A clean setup does not guarantee a clean run. The "romance" of engineering lies in observation—watching the bubble formation on an electrode, noting the subtle shift in electrolyte color, or sensing a temperature spike.
These are the vital signs of the experiment.
Additionally, the apparatus itself—often fragile glass—must be respected. A micro-crack in a cell body is a structural failure that invites leaks and safety hazards. Gentle handling is a technical skill.
Summary: The Hierarchy of Controls
To secure your results, you must operationalize your defense against contamination.
| Threat Vector | The "Why" (Risk) | The "How" (Mitigation) |
|---|---|---|
| Particulates | Dust introduces rogue ions/reaction sites. | Isolate the workspace from airborne debris. |
| Biological | Skin oils insulate electrodes and skew data. | Enforce a strict "No-Touch" protocol. |
| Chemical | Byproducts re-enter the reaction loop. | Ensure dynamic ventilation. |
| Thermal | Heat alters conductivity and safety profiles. | Remove flammables; monitor temperature. |
| Structural | Cracks/breaks cause leaks and hazards. | Handle glass apparatus with extreme care. |
Precision Requires the Right Foundation
You can have the most disciplined protocol in the world, but if your hardware introduces impurities, the math will never work.
KINTEK specializes in lab equipment and consumables built for the rigor of modern electrochemistry. We understand that in an electrolytic cell, the material is the method.
We help you eliminate variables by providing:
- High-Purity Materials: Electrodes and cells designed to minimize background interference.
- Engineered Durability: Apparatus built to withstand handling while maintaining structural integrity.
- Consistency: Consumables that perform exactly the same way, every single time.
When you control the environment, the procedure, and the equipment, you stop fighting the noise and start hearing the signal.
Ready to secure your experimental integrity? Contact Our Experts to discuss how KINTEK can upgrade your laboratory standards.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Side Window Optical Electrolytic Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Thin-Layer Spectral Electrolysis Electrochemical Cell
Related Articles
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- Handheld Coating Thickness Gauges: Accurate Measurement for Electroplating and Industrial Coatings
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Glass Heart: Why Good Science Dies in Dirty Cells








