Basic Concepts of Pressure
Normal Pressure
Normal pressure, also known as atmospheric pressure, is the pressure exerted by the Earth's atmosphere at any given point. This pressure is typically measured at sea level and is approximately 101,325 Pascals (Pa) or 100 Kilopascals (KPa). This standard value serves as a reference point for various pressure measurements and calculations.
Atmospheric pressure can vary slightly depending on factors such as altitude, weather conditions, and geographic location. For instance, at higher altitudes, the atmospheric pressure is lower because there is less air above exerting force. Conversely, during certain weather patterns, such as a high-pressure system, the atmospheric pressure can be slightly higher than the standard value.
Understanding normal pressure is crucial in many scientific and engineering applications. It serves as a baseline for measuring both positive and negative pressures. Positive pressure, which exceeds normal atmospheric pressure, is commonly used in scenarios like inflating tires or pressurizing water systems. On the other hand, negative pressure, or vacuum, which is below atmospheric pressure, finds applications in processes such as vacuum sealing or medical suction.
In summary, normal pressure is a fundamental concept that provides a reference for various pressure-related phenomena and applications. Its standard value of 101,325 Pa or 100 KPa at sea level is widely used across different fields to ensure consistency and accuracy in pressure measurements and calculations.

Negative Pressure (Vacuum)
Negative pressure, commonly referred to as vacuum, is a condition where the pressure within a system is lower than the surrounding atmospheric pressure. This concept is fundamental in various scientific and engineering applications, ranging from simple everyday activities to complex industrial processes.
One of the most familiar examples of negative pressure is the act of drinking through a straw. When you suck on a straw, you create a partial vacuum inside it, which allows the liquid to be drawn up from the container. This is because the pressure inside the straw drops below the atmospheric pressure outside, causing the liquid to flow upwards.
In more advanced applications, negative pressure is harnessed in vacuum pumps, which are essential tools in scientific research, biological engineering, and environmental protection. These pumps create a vacuum by removing gas molecules from a sealed chamber, thereby reducing the pressure inside. This process is crucial for gas sampling, circulation, and numerous other applications where controlling pressure is key.
The measurement of negative pressure can be relative or absolute. Relative pressure measures the difference between the pressure in the system and the local atmospheric pressure, while absolute pressure measures the pressure relative to a perfect vacuum. The relationship between these two measurements is given by the formula:
[ \text{Relative pressure} = \text{Absolute pressure} - \text{Local atmospheric pressure} ]
Understanding these principles is essential for anyone working with pressure systems, as it enables precise control and manipulation of pressure conditions for various practical applications.

Positive Pressure
Positive pressure, as the name suggests, is the condition where the pressure within a system exceeds the atmospheric pressure. This concept is fundamental in various applications, particularly in industrial and mechanical settings. For instance, when inflating tires, the pressure inside the tire must be higher than the atmospheric pressure to ensure proper inflation and functionality.
Positive pressure is not limited to just tire inflation; it plays a crucial role in numerous other scenarios. In medical applications, positive pressure ventilation systems are used to assist patients with breathing difficulties. These systems deliver air or oxygen at a pressure higher than the surrounding atmosphere, ensuring a steady flow of air into the lungs.
In industrial processes, positive pressure is often employed to maintain controlled environments, such as cleanrooms in semiconductor manufacturing. By maintaining a higher pressure inside these rooms, the risk of contamination from external particles is significantly reduced.
| Application | Description |
|---|---|
| Tire Inflation | Ensures proper tire pressure for safe and efficient vehicle operation. |
| Medical Ventilation | Assists patients with breathing difficulties by delivering air at higher pressure. |
| Cleanrooms | Maintains a controlled environment by keeping pressure higher than outside. |
Understanding positive pressure is essential for engineers, technicians, and anyone involved in pressure-related applications. It ensures the safety, efficiency, and reliability of systems and processes that rely on maintaining pressure differentials.
Applications and Parameters of Vacuum Pumps
Vacuum in Various Fields
Vacuum pumps are indispensable tools across a spectrum of scientific disciplines, each leveraging their unique capabilities to manipulate and analyze gases in controlled environments. In scientific research, these pumps facilitate the creation of ultra-low pressure conditions necessary for experiments in particle physics, material science, and space simulation. They ensure the purity and stability of the experimental environment, allowing researchers to draw precise conclusions from their studies.
In biological engineering, vacuum pumps play a crucial role in processes such as cell culture, sterilization, and the production of pharmaceuticals. By maintaining a controlled vacuum, these pumps help in the efficient circulation of gases, ensuring optimal growth conditions for microorganisms and cells. This precision is vital for the development of life-saving drugs and medical treatments.

Environmental protection also benefits significantly from vacuum technology. Vacuum pumps are employed in gas sampling and analysis to monitor air quality, detect pollutants, and study the effects of industrial emissions. By accurately controlling the pressure and gas flow, these pumps enable scientists to gather reliable data, which is essential for formulating effective environmental policies and mitigation strategies.
The versatility of vacuum pumps extends beyond these fields, showcasing their importance in advancing knowledge and technology across various sectors.
Types of Pressure Measurement
Pressure measurement can be categorized into two primary types: absolute pressure and relative pressure. Each type serves distinct purposes and is used in different contexts to provide accurate readings.
Absolute Pressure: This type of pressure measurement is taken relative to an absolute vacuum. An absolute vacuum represents a state where no pressure exists, making it the zero point on the absolute pressure scale. Instruments that measure absolute pressure are often used in scientific research and industrial applications where precise pressure readings are crucial. For instance, in aerospace engineering, absolute pressure sensors are essential for monitoring cabin pressure in aircraft.
Relative Pressure: Also known as gauge pressure, relative pressure is measured relative to the atmospheric pressure. Atmospheric pressure, which is approximately 101.325 kPa at sea level, serves as the baseline for relative pressure measurements. Devices that measure relative pressure are commonly used in everyday applications such as tire pressure gauges and blood pressure monitors. In these cases, the pressure is gauged against the ambient atmospheric pressure, providing a practical reading for immediate use.
Understanding the difference between these two types of pressure measurements is fundamental for accurate pressure readings across various fields, from scientific research to everyday applications.
Conversion Between Absolute and Relative Pressure
Understanding the relationship between absolute and relative pressure is crucial for various applications, especially in scientific and engineering contexts. The conversion between these two types of pressure is straightforward and can be summarized by a simple equation:
Relative pressure = Absolute pressure - Local atmospheric pressure
To delve deeper into this concept, let's break down the components involved:
-
Absolute Pressure: This is the pressure measured relative to a perfect vacuum. It represents the total pressure exerted by a system, including the pressure exerted by the atmosphere.
-
Local Atmospheric Pressure: This refers to the pressure exerted by the atmosphere at a specific location. It varies depending on altitude, weather conditions, and other environmental factors.
-
Relative Pressure: Also known as gauge pressure, this is the pressure measured relative to the local atmospheric pressure. It indicates the difference between the absolute pressure and the local atmospheric pressure.
This conversion is particularly useful in scenarios where the actual pressure in a system needs to be determined relative to the ambient conditions. For instance, in industrial applications such as inflating tires or operating vacuum pumps, knowing the relative pressure can provide critical insights into the performance and safety of the system.
For example, if the absolute pressure in a tire is measured at 300 kPa and the local atmospheric pressure is 100 kPa, the relative pressure would be:
Relative pressure = 300 kPa - 100 kPa = 200 kPa
This calculation helps engineers and technicians understand the effective pressure within the tire, which is essential for maintaining optimal performance and safety.
In summary, the conversion between absolute and relative pressure is a fundamental concept that bridges theoretical measurements with practical applications. By understanding this relationship, professionals can better manage and control pressure in various systems, ensuring efficiency and safety in their operations.
Unit Conversions and Practical Examples
Pressure Unit Conversion
Understanding the conversion factors between different pressure units is crucial for accurate measurements and comparisons across various fields. The most commonly used pressure units include Pascals (Pa), Kilogram force per square centimeter (Kgf/cm²), bars (bar), atmospheres (atm), torrs (Torr), and pounds per square inch (PSI).
| Unit | Symbol | Conversion to Pascals (Pa) |
|---|---|---|
| Pascal | Pa | 1 Pa |
| Kilogram force per square centimeter | Kgf/cm² | 98066.5 Pa |
| Bar | bar | 100,000 Pa |
| Atmosphere | atm | 101,325 Pa |
| Torr | Torr | 133.322 Pa |
| PSI | PSI | 6,894.76 Pa |
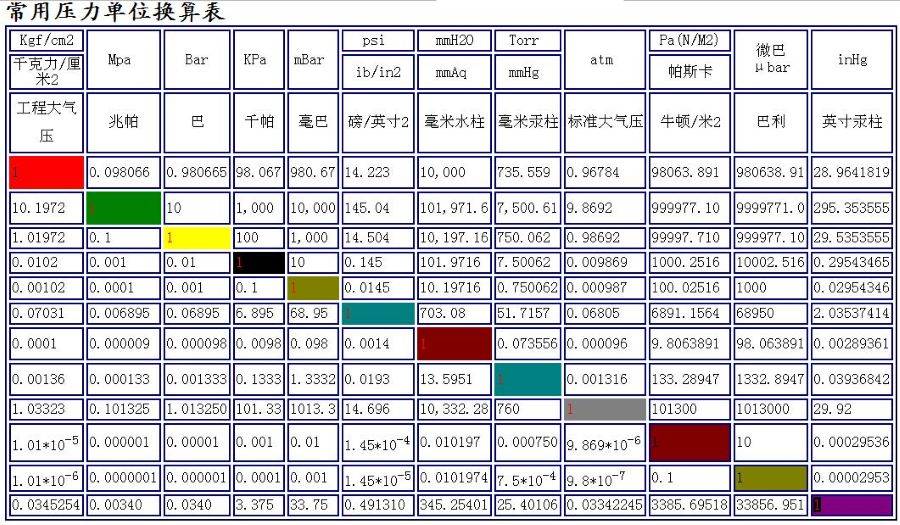 For instance, converting 1 bar to Pascals involves multiplying by 100,000, while converting 1 atm to Pascals requires multiplying by 101,325. These conversions are essential for ensuring consistency in pressure measurements, whether in scientific research, industrial applications, or everyday tasks like checking tire pressure.
For instance, converting 1 bar to Pascals involves multiplying by 100,000, while converting 1 atm to Pascals requires multiplying by 101,325. These conversions are essential for ensuring consistency in pressure measurements, whether in scientific research, industrial applications, or everyday tasks like checking tire pressure.
By mastering these conversion factors, professionals and enthusiasts alike can seamlessly navigate between different pressure units, facilitating better understanding and more precise control in their respective fields.
Example of Pressure Application
To illustrate the impact of different vacuum pumps on the pressure within a closed container, let's consider a practical scenario. Imagine a sealed container initially at atmospheric pressure, which is approximately 101325 Pa or 100 kPa. When a diaphragm vacuum pump is connected to this container, it begins to remove air molecules, thereby reducing the internal pressure.
| Pump Type | Pressure Change |
|---|---|
| Rotary Vane Pump | Reduces pressure to around 10^-2 to 10^-3 Pa, creating a significant vacuum. |
| Diaphragm Pump | Achieves pressures around 10^-1 to 10^0 Pa, suitable for moderate vacuum needs. |
| Turbomolecular Pump | Can reach ultra-high vacuum levels, down to 10^-9 Pa, ideal for scientific research. |
This process demonstrates the transition from positive pressure (atmospheric) to negative pressure (vacuum) as air is evacuated. Conversely, if a compressor is used instead of a lab vacuum pump, the pressure inside the container would increase, moving from atmospheric pressure towards higher positive pressure. This shift highlights the fundamental concepts of positive and negative pressure, showcasing how external devices can manipulate internal conditions within a sealed environment.
Related Products
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Laboratory Vertical Water Circulating Vacuum Pump for Lab Use
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use














