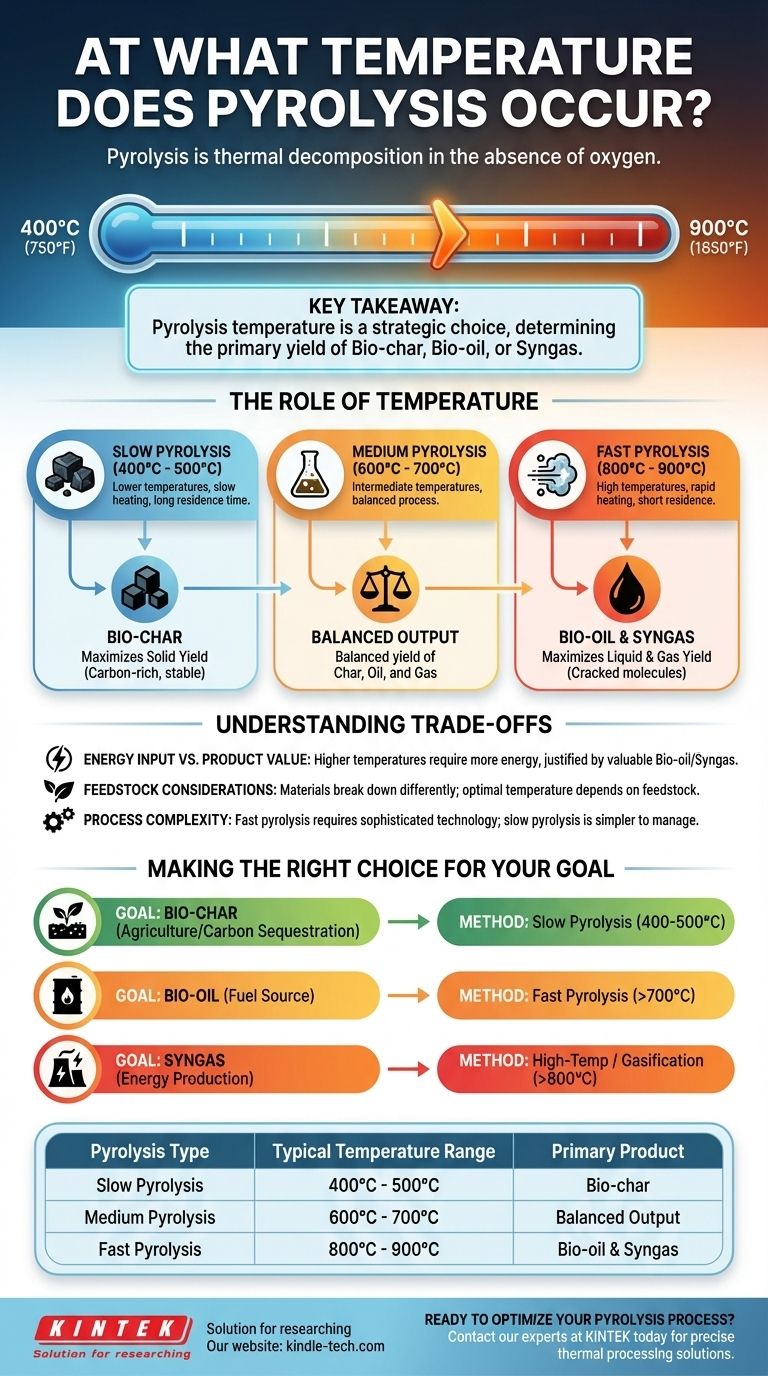
Pyrolysis is a process of thermal decomposition that occurs in the absence of oxygen. While there is no single temperature for this process, it generally takes place in a range between 400°C and 900°C (750°F to 1650°F). The precise temperature is a critical control parameter that depends entirely on the material being processed and the desired end products.
The key takeaway is that pyrolysis temperature is not a fixed point, but a strategic choice. This choice directly determines whether the process will primarily yield solid charcoal, liquid bio-oil, or combustible gas.

The Role of Temperature in Pyrolysis
Pyrolysis is essentially the act of breaking down complex materials with heat, without allowing them to combust. The temperature at which this happens dictates both the speed of the reaction and the nature of the output.
The Three Core Products
Regardless of the temperature, pyrolysis breaks down organic feedstock into three main products:
- Bio-char: A stable, solid, carbon-rich material similar to charcoal.
- Bio-oil: A dense, acidic liquid, also known as pyrolysis oil.
- Syngas: A mixture of combustible gases, including hydrogen, carbon monoxide, and methane.
How Temperature Shifts the Output
The balance of these three products is almost entirely controlled by temperature and the rate of heating. This leads to different classifications of the process.
Slow Pyrolysis (Maximizing Bio-char)
This process uses lower temperatures, typically around 400°C to 500°C, and a very slow heating rate. The longer residence time allows for the maximum conversion of the feedstock into bio-char.
Medium Pyrolysis (Balanced Output)
Operating in a range of approximately 600°C to 700°C, this method provides a more balanced yield of char, oil, and gas. It serves as an intermediate process between the two extremes.
Fast Pyrolysis (Maximizing Bio-oil and Syngas)
This method uses high temperatures, often between 800°C and 900°C, combined with a very rapid heating rate. These conditions "crack" the molecules quickly, minimizing char formation and maximizing the production of bio-oil and syngas.
Understanding the Trade-offs
Choosing the right temperature is a balancing act between energy costs, equipment complexity, and the value of the final products.
Energy Input vs. Product Value
Higher temperatures require a significantly greater energy input to maintain. This cost may only be justified if the resulting bio-oil or syngas is more valuable or useful for a specific application than the bio-char produced at lower temperatures.
Feedstock Considerations
Different materials, or feedstocks, break down at different rates. For example, woody biomass may pyrolyze differently than agricultural waste or plastics, requiring adjustments to the optimal temperature range to achieve the desired outcome.
Process Complexity
Fast pyrolysis, while effective for producing liquids and gases, requires more sophisticated and precisely controlled reactor technology. Slow pyrolysis is generally a simpler and more robust process to manage.
Making the Right Choice for Your Goal
The optimal pyrolysis temperature is defined by your primary objective.
- If your primary focus is creating bio-char for agriculture or carbon sequestration: A low-temperature (400-500°C), slow pyrolysis process is the most direct and efficient method.
- If your primary focus is producing liquid bio-oil as a potential fuel source: A high-temperature (above 700°C), fast pyrolysis process is necessary to maximize liquid yields.
- If your primary focus is generating syngas for energy production: The highest temperatures (above 800°C), often in a process called gasification (pyrolysis with a limited oxidant), are most effective.
Ultimately, temperature is the fundamental lever you use to steer the outcome of the pyrolysis reaction.
Summary Table:
| Pyrolysis Type | Typical Temperature Range | Primary Product |
|---|---|---|
| Slow Pyrolysis | 400°C - 500°C | Bio-char |
| Medium Pyrolysis | 600°C - 700°C | Balanced Output |
| Fast Pyrolysis | 800°C - 900°C | Bio-oil & Syngas |
Ready to Optimize Your Pyrolysis Process?
Choosing the right temperature is critical for achieving your desired product yield. KINTEK specializes in high-quality lab equipment and consumables for precise thermal processing. Whether you're researching slow pyrolysis for bio-char or developing fast pyrolysis systems for bio-oil, our reliable furnaces and reactors provide the control and durability you need.
Let's discuss your specific application. Contact our experts today to find the perfect pyrolysis solution for your laboratory.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
People Also Ask
- What is the difference between pyrolysis and incineration? Material Recovery vs. Energy Recovery
- How to measure the thickness of coating? Choose the Right Method for Your Substrate
- How is filter press capacity calculated? Unlock Accurate Sizing for Your Specific Slurry
- How much sample is needed for IR? Optimize Your Analysis with Minimal Material
- Which method cannot be used for carbon nanotube production? Understand the Key Synthesis Principles
- What are the principles of physical vapor deposition of thin films? Master the 3-Step Process for High-Purity Coatings
- What is the principle of wiped film molecular still? Preserve and Purify Heat-Sensitive Compounds
- What are the yields of pyrolysis? A Guide to Maximizing Biochar, Bio-oil, or Syngas



















