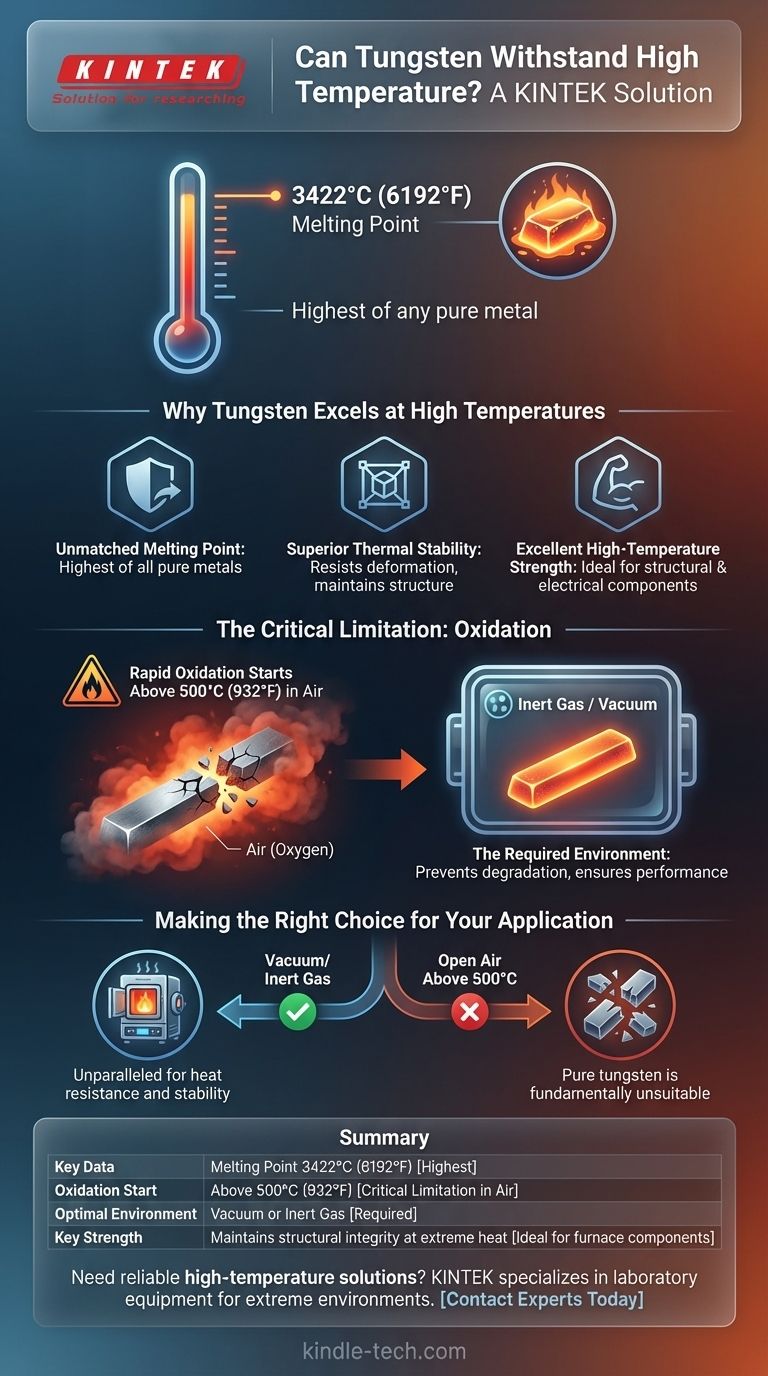
Yes, tungsten is renowned for its exceptional ability to withstand high temperatures. It possesses the highest melting point of any pure metal, 3422°C (6192°F), making it a cornerstone material for applications involving extreme heat. However, its performance is critically dependent on the surrounding atmosphere.
Tungsten's extraordinary heat resistance is only realized in a vacuum or an inert gas environment. In the presence of oxygen, it begins to oxidize and degrade rapidly at temperatures far below its melting point.

Why Tungsten Excels at High Temperatures
Tungsten's unique atomic structure gives it several properties that make it ideal for high-heat scenarios, provided the environmental conditions are correct.
Unmatched Melting Point
The most defining characteristic of tungsten is its melting point of 3422°C (6192°F). This is the highest of all pure metals, placing it in a class of its own for thermal endurance.
Superior Thermal Stability
Even at temperatures well below its melting point, tungsten maintains its structural integrity and strength. This high thermal stability means it resists deforming or weakening when subjected to intense heat.
Excellent High-Temperature Strength
Beyond simply not melting, tungsten has excellent strength at elevated temperatures. This allows it to function as a structural or electrical component in environments where most other metals would have failed.
The Critical Limitation: Oxidation
Understanding tungsten's primary weakness is essential for its successful implementation. Its remarkable properties are quickly negated by a single environmental factor: oxygen.
The Problem with Oxygen
When exposed to air, tungsten begins to oxidize rapidly at temperatures above 500°C (932°F). This is a crucial detail, as this temperature is more than 2900°C below its actual melting point.
What Oxidation Means
This is not a passive surface discoloration. The oxidation is an aggressive chemical reaction that forms a brittle oxide layer, causing the material to degrade and lose its structural integrity, leading to component failure.
The Required Environment
To prevent this degradation, tungsten parts must be operated in a vacuum or an inert (non-reactive) atmosphere. Gases like argon are commonly used to create a protective environment that allows the metal to function at extreme temperatures without oxidizing.
Making the Right Choice for Your Application
Your decision to use tungsten should be based entirely on the operating environment of your component.
- If your primary focus is on applications in a vacuum or inert gas: Tungsten is an unparalleled choice for its heat resistance, high-temperature strength, and stability.
- If your primary focus is on applications in open air above 500°C: Pure tungsten is fundamentally unsuitable and will fail; you must consider alternative materials or specialized tungsten alloys designed for oxidation resistance.
Ultimately, tungsten's suitability for a high-temperature role is determined not just by its melting point, but by your ability to protect it from oxygen.
Summary Table:
| Property | Value | Key Consideration |
|---|---|---|
| Melting Point | 3422°C (6192°F) | Highest of all pure metals |
| Oxidation Start | Above 500°C (932°F) | Critical limitation in air |
| Optimal Environment | Vacuum or Inert Gas | Required for high-temperature performance |
| Key Strength | Maintains structural integrity at extreme heat | Ideal for vacuum furnace components |
Need reliable high-temperature solutions for your lab? KINTEK specializes in laboratory equipment and consumables designed for extreme environments. Our tungsten components and furnace systems are engineered to deliver superior performance in vacuum and inert gas applications. Contact our experts today to discuss how our high-temperature solutions can enhance your laboratory's capabilities and ensure your materials withstand the most demanding thermal conditions.
Visual Guide
Related Products
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- High Temperature Wear-Resistant Alumina Al2O3 Plate for Engineering Advanced Fine Ceramics
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
People Also Ask
- What does a hydraulic heat press do? Achieve Industrial-Scale, Consistent Pressure for High-Volume Production
- What are heated hydraulic presses used for? Molding Composites, Vulcanizing Rubber, and More
- Why do you need to follow the safety procedure in using hydraulic tools? Prevent Catastrophic Failure and Injury
- Does a hydraulic press have heat? How Heated Platens Unlock Advanced Molding and Curing
- What is a hot hydraulic press? Harness Heat and Pressure for Advanced Manufacturing



















