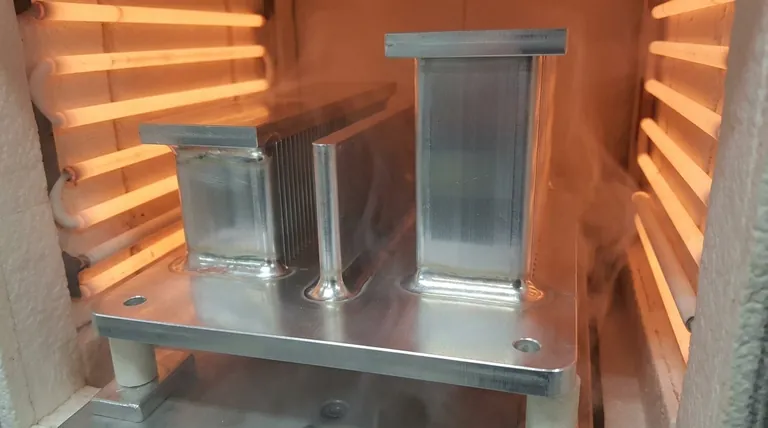
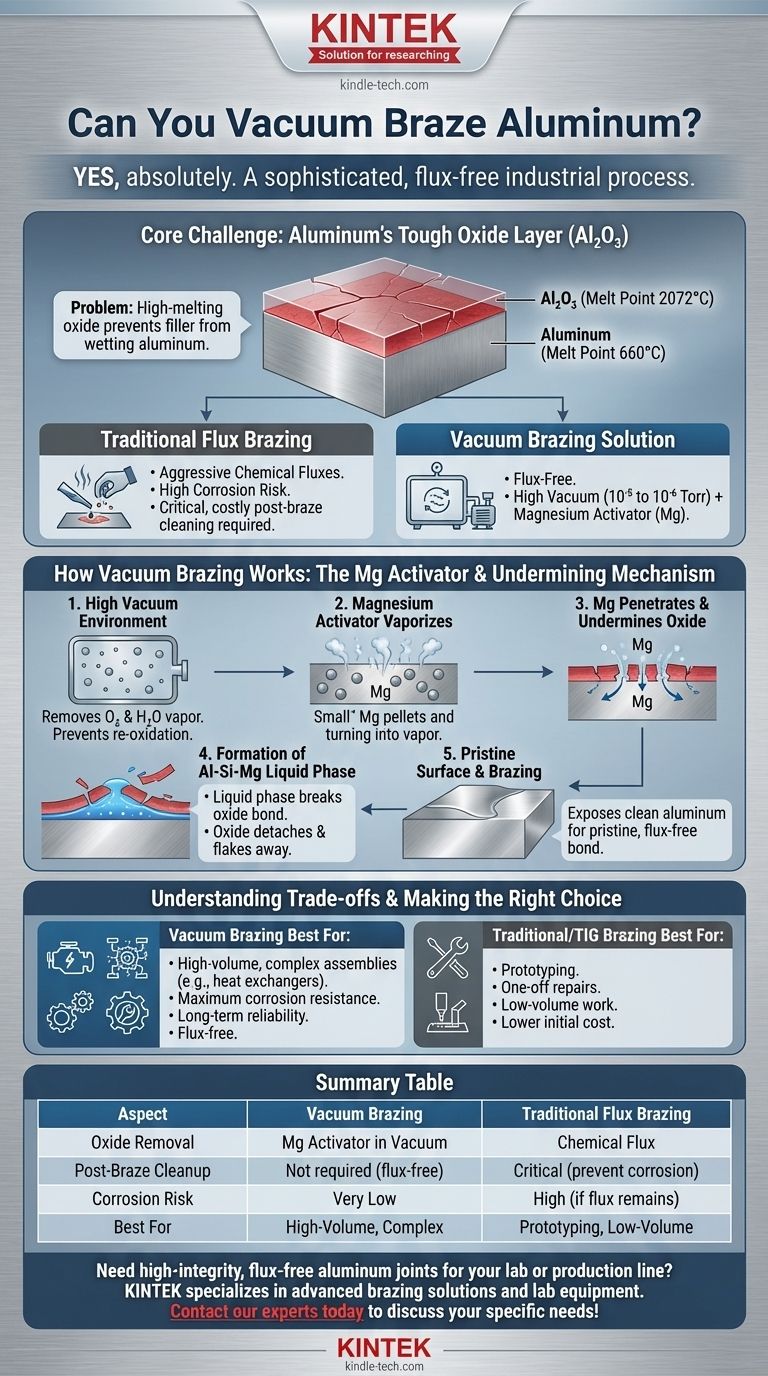
Yes, you can absolutely vacuum braze aluminum. It is a sophisticated and highly effective industrial process used to create strong, clean joints without the need for chemical fluxes. The method relies on a high-vacuum environment combined with a metal activator, typically magnesium, to chemically remove the stubborn oxide layer that naturally forms on aluminum surfaces, allowing the brazing filler metal to properly wet and bond with the base material.
Brazing aluminum is fundamentally a battle against its tough, passive oxide layer (Al₂O₃). Vacuum brazing wins this battle not by force, but with finesse—using a high vacuum and a magnesium activator to undermine and detach the oxide film from beneath the surface, enabling a pristine, flux-free bond.
The Core Challenge: Aluminum's Oxide Layer
The primary obstacle in joining aluminum is not the metal itself, but the thin, transparent layer of aluminum oxide (Al₂O₃) that instantly forms on its surface when exposed to air.
Why Al₂O₃ is a Problem
This oxide layer is incredibly tenacious and stable. It melts at approximately 2072°C (3762°F), while aluminum itself melts at a much lower 660°C (1220°F).
During brazing, the filler metal must melt and flow onto the base metal. The high-melting-point oxide film acts as a barrier, preventing the molten filler from making contact and "wetting" the aluminum surface, thus inhibiting a proper metallurgical bond.
The Traditional Solution (and its Drawbacks)
Historically, this problem was solved using aggressive chemical fluxes. These fluxes would chemically attack and dissolve the oxide layer, but they are highly corrosive.
If not completely removed after brazing, residual flux can become trapped in the joint, leading to catastrophic corrosion and component failure over time. This makes post-braze cleaning a critical, costly, and difficult step.
How Vacuum Brazing Solves the Oxide Problem
Vacuum brazing provides an elegant, flux-free solution by altering the chemical environment inside a sealed furnace.
The Role of the Vacuum
The process is performed in a high vacuum, typically in the 10⁻⁵ to 10⁻⁶ Torr range. This low-pressure environment removes virtually all oxygen and water vapor from the furnace chamber.
By eliminating these reactive gases, the vacuum prevents the aluminum from re-oxidizing as it is heated toward its brazing temperature.
The Key Ingredient: The Magnesium Activator
With oxidation prevented, the final challenge is to remove the pre-existing oxide film. This is accomplished by adding a small amount of an activator, most commonly magnesium (Mg), into the furnace, often as part of the brazing filler material.
Early theories suggested magnesium simply "scavenged" any remaining oxygen. However, the true mechanism is more sophisticated.
The Undermining Mechanism
As the furnace heats up, the magnesium vaporizes. This Mg vapor penetrates through microscopic cracks and defects in the Al₂O₃ layer.
Once beneath the oxide film, the magnesium reacts with the base aluminum and silicon (from the filler metal) to form a low-melting-point Al-Si-Mg liquid phase right at the interface.
This liquid phase melts before the main filler alloy, effectively breaking the bond between the oxide film and the base aluminum. The oxide film detaches and flakes away, exposing a perfectly clean, raw aluminum surface for the brazing filler to bond with.
Understanding the Trade-offs
While powerful, vacuum brazing is not a universal solution. It involves specific equipment and process considerations.
High Initial Investment
Vacuum brazing furnaces are complex and represent a significant capital investment compared to the equipment needed for conventional torch or induction brazing.
Process Expertise Required
The process requires precise control over temperature profiles, vacuum levels, and material chemistry. It is a highly engineered process best suited for controlled, repeatable production environments.
Material and Design Constraints
Not all aluminum alloys are suitable for vacuum brazing. The process works best with specific "brazeable" alloys and clad materials. Part design must also allow the magnesium vapor to reach all joint areas.
Making the Right Choice for Your Application
Deciding whether to specify vacuum brazing depends entirely on your project's goals, scale, and performance requirements.
- If your primary focus is high-volume production of complex assemblies (like automotive heat exchangers or aerospace cold plates): Vacuum brazing is the industry standard for its unmatched repeatability and ability to produce clean, reliable, flux-free joints in batch processes.
- If your primary focus is maximum corrosion resistance and long-term reliability: Vacuum brazing is the superior choice because it completely eliminates the risk of trapped corrosive flux, which is a common failure mode in flux-brazed components.
- If your primary focus is prototyping, one-off repairs, or low-volume work: The high cost and complexity of vacuum brazing make it impractical. Traditional TIG brazing or modern flux-based methods are far more accessible and cost-effective.
By understanding the underlying science, you can confidently leverage vacuum brazing for applications that demand the highest level of quality and performance.
Summary Table:
| Aspect | Vacuum Brazing | Traditional Flux Brazing |
|---|---|---|
| Oxide Removal | Magnesium activator in a vacuum | Chemical flux |
| Post-Braze Cleanup | Not required (flux-free) | Critical to prevent corrosion |
| Corrosion Risk | Very low | High if flux is not fully removed |
| Best For | High-volume, complex assemblies (e.g., heat exchangers) | Prototyping, low-volume repairs |
Need high-integrity, flux-free aluminum joints for your lab or production line? KINTEK specializes in advanced brazing solutions and lab equipment. Our expertise ensures your aluminum assemblies achieve maximum strength and corrosion resistance. Contact our experts today to discuss how we can support your specific laboratory and manufacturing needs!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1200℃ Muffle Furnace Oven for Laboratory
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
People Also Ask
- What are the advantages of a tube furnace? Achieve Superior Thermal Control and Purity
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness
- What factors influence the general design of a tube furnace? Match Your Process with the Perfect System
- What is the function of a tube furnace? Achieve Precise High-Temperature Processing in a Controlled Atmosphere
- What are the benefits of a tube furnace? Achieve Superior Temperature & Atmosphere Control


















