In short, the accuracy of X-ray fluorescence (XRF) analysis is not one single value. It is highly variable, ranging from a few parts per million (ppm) for heavy elements in an ideal sample, to several weight percent (wt%) for light elements in a complex, unprepared sample. True accuracy depends entirely on the element being measured, the instrument being used, the quality of the calibration, and how the sample is prepared.
XRF is fundamentally a comparative technique, not an absolute one. Its accuracy is therefore limited by how closely the calibration standards match the chemical and physical properties of the unknown sample being analyzed. Think of it less as a perfect scale and more as a highly sophisticated tool for comparison.

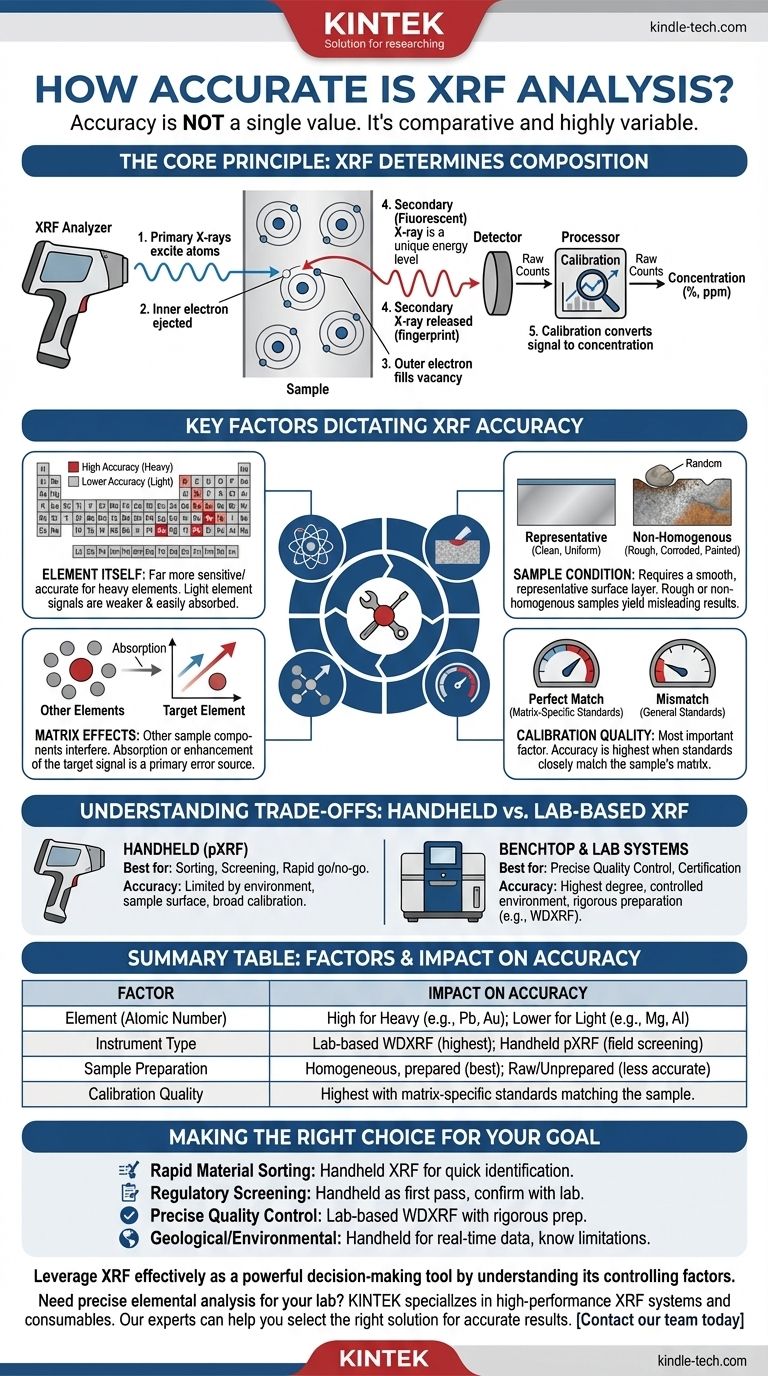
The Core Principle: How XRF Determines Composition
To understand XRF's accuracy, you must first understand how it works. The process is based on a predictable atomic-level interaction.
Primary vs. Secondary X-rays
An XRF analyzer fires a beam of primary X-rays at a sample. This energy excites the atoms within the material, causing them to eject an inner-shell electron.
This creates an unstable vacancy, which is immediately filled by an outer-shell electron. As this electron "falls" into the lower energy state, it releases a secondary, or fluorescent, X-ray.
From Signal to Concentration
The energy of this secondary X-ray is a unique "fingerprint" for each element. The instrument's detector counts the number of fingerprints for each element.
Crucially, the instrument then uses a pre-loaded calibration model to convert these raw counts into a concentration, like percent (%) or parts per million (ppm). This calibration step is the root of most accuracy-related questions.
Key Factors That Dictate XRF Accuracy
The final accuracy you achieve is a result of several interacting factors. A change in any one of these can significantly alter your results.
The Element Itself (Atomic Number)
XRF is far more sensitive and accurate for heavy elements (like lead, mercury, or gold) than for light elements (like magnesium, aluminum, or silicon).
This is because light elements emit low-energy secondary X-rays that are easily absorbed by the air or by other elements in the sample itself. They produce a weaker signal that is harder to detect and quantify reliably.
Sample Homogeneity and Surface Condition
XRF only analyzes a very thin surface layer of the sample. For an accurate reading, this surface must be representative of the entire material.
A rough, corroded, dusty, or painted surface will produce misleading results. Likewise, a non-homogenous sample—like a soil sample with a random pebble of a high-concentration mineral—can skew the reading dramatically.
Matrix Effects
The "matrix" refers to everything in the sample that is not the specific element you are trying to measure. These other elements can interfere with the analysis.
Absorption-enhancement effects are the most common issue. The signal from your element of interest can be absorbed by another element before it reaches the detector (reducing its apparent concentration) or enhanced by the fluorescence of another element (increasing its apparent concentration). This is a primary source of analytical error.
The Quality of Calibration
This is the single most important factor. Since XRF is a comparative method, its accuracy is only as good as the reference materials used to create its calibration.
If you are analyzing a specific stainless steel alloy, a calibration built using certified stainless steel standards will be highly accurate. If you try to use a general "metals" or "soil" calibration for that same sample, the results will be significantly less accurate due to different matrix effects.
Understanding the Trade-offs: Handheld vs. Lab-Based XRF
The term "XRF" can refer to very different classes of instruments, each with its own accuracy profile.
Handheld (pXRF)
Handheld analyzers are designed for speed, portability, and convenience. They are exceptional tools for sorting, screening, and qualitative or semi-quantitative identification in the field.
However, their accuracy is inherently limited by environmental factors, variable sample surfaces, and typically broader calibration models. They are best used for rapid go/no-go decisions.
Benchtop and Lab Systems (WDXRF/EDXRF)
Larger, lab-based systems offer a much higher degree of accuracy and precision. They operate in a controlled environment and are used with carefully prepared samples (e.g., fused beads or pressed pellets).
Wavelength Dispersive XRF (WDXRF), in particular, offers superior resolution and sensitivity, especially for light elements. These instruments are the choice for process control and certification where the highest accuracy is non-negotiable.
Making the Right Choice for Your Goal
To get the most out of XRF, match the method and preparation level to your specific objective.
- If your primary focus is rapid material sorting (e.g., scrap metal, alloy verification): A handheld XRF is the perfect tool; absolute ppm accuracy is less important than making a correct identification quickly.
- If your primary focus is regulatory screening (e.g., RoHS, consumer product safety): Handheld XRF is an excellent and cost-effective first pass, but be prepared to confirm failing results with a certified lab using a more definitive method.
- If your primary focus is precise quality control (e.g., cement, mining, or alloy production): A lab-based WDXRF system with matrix-specific calibrations and rigorous sample preparation is the only way to achieve the required high accuracy.
- If your primary focus is geological or environmental field screening: A handheld XRF with a relevant calibration (e.g., "soil") provides invaluable real-time data, but you must understand its limitations regarding moisture, surface texture, and light elements.
By understanding these controlling factors, you can effectively leverage XRF not just as a measurement device, but as a powerful decision-making tool.
Summary Table:
| Factor | Impact on Accuracy |
|---|---|
| Element (Atomic Number) | High accuracy for heavy elements (e.g., Pb, Au); lower for light elements (e.g., Mg, Al) |
| Instrument Type | Lab-based WDXRF offers highest accuracy; handheld pXRF is ideal for field screening/sorting |
| Sample Preparation | Homogeneous, prepared samples (e.g., pressed pellets) yield far more accurate results than raw/unprepared samples |
| Calibration Quality | Accuracy is highest when using matrix-specific standards that closely match the unknown sample |
Need precise elemental analysis for your lab? KINTEK specializes in high-performance lab equipment, including XRF systems and consumables for sample preparation. Whether you require the ultimate accuracy of a benchtop WDXRF for quality control or the portability of a handheld analyzer for field screening, our experts can help you select the right solution. Contact our team today to discuss your specific application and ensure your analytical results are accurate and reliable.
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
People Also Ask
- What is the maximum temperature for all metal hot end? It's not the metal, it's the electronics.
- What is the primary function of a high-speed magnetic stirrer in the synthesis of Pd-on-Au NPs? Ensure Uniform Diffusion
- What is the difference between biomass briquettes and pellets? Choose the Right Fuel for Your Heating System
- What is the process of making bio-oil? Convert Biomass to Liquid Fuel via Pyrolysis
- What are the grades of graphite? A Practical Guide to Choosing the Right Material for Your Application
- Why is an ultrasonic cleaner used with ethanol to treat alloy specimens? Ensure Superior Diffusion Bonding Results
- What is magnetron sputtering target? The Core Material for High-Performance Thin Film Coatings
- What are the precautions to be taken while sampling? Ensure Data Accuracy and Minimize Bias










