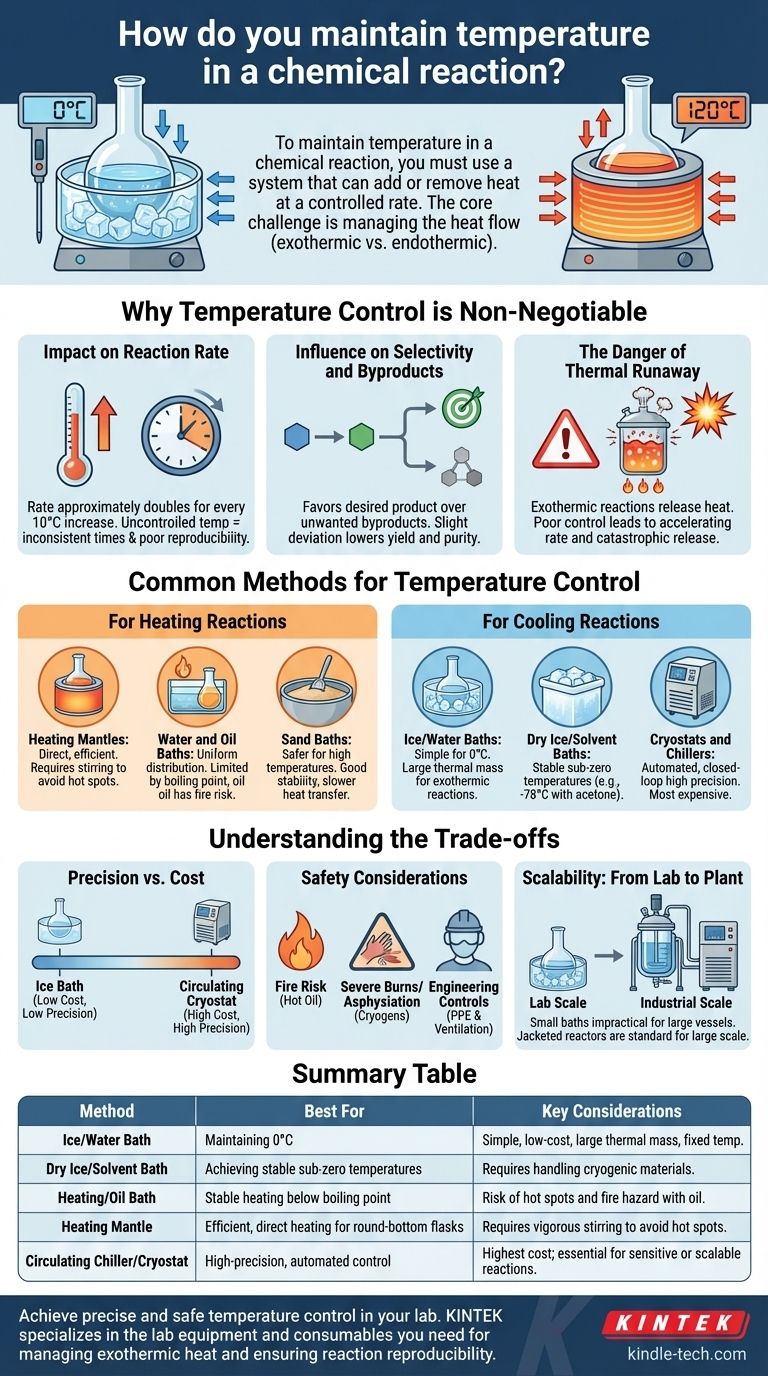
To maintain temperature in a chemical reaction, you must use a system that can add or remove heat at a controlled rate. The most common methods involve immersing the reaction vessel in a thermal bath (like water, oil, or a cryogenic slurry) or using equipment like heating mantles, chillers, and jacketed reactors that circulate a temperature-controlled fluid.
The core challenge of temperature control is not just setting a temperature, but actively managing the heat flow. Every reaction is either exothermic (releasing heat) or endothermic (absorbing heat), and the chosen method must be able to counteract this thermal effect to maintain a stable environment.

Why Temperature Control is Non-Negotiable
Failing to control temperature is one of the most common reasons for a failed reaction. It directly governs the speed, outcome, and safety of your chemical transformation.
Impact on Reaction Rate
According to fundamental chemical kinetics, the rate of a reaction is highly dependent on temperature. A general rule of thumb is that the reaction rate approximately doubles for every 10°C increase.
Uncontrolled temperature leads to inconsistent reaction times and makes the process impossible to reproduce reliably.
Influence on Selectivity and Byproducts
Many reactions can proceed down multiple pathways, leading to different products. Temperature is often the deciding factor that favors the formation of your desired product over unwanted byproducts.
Even a slight deviation from the optimal temperature can dramatically lower your reaction's yield and purity, complicating the subsequent purification process.
The Danger of Thermal Runaway
For exothermic reactions, which release heat, poor temperature control can be catastrophic. If heat is generated faster than it can be removed, the reaction temperature will rise, which in turn accelerates the reaction rate, releasing even more heat.
This vicious cycle is known as thermal runaway and can lead to violent boiling, pressure buildup, and even explosions.
Common Methods for Temperature Control
The right tool depends on your target temperature, the scale of your reaction, and the level of precision required.
For Heating Reactions
Heating mantles are fabric-like shells containing electrical heating elements. They are shaped to fit round-bottom flasks, providing efficient and direct heat. However, they can create localized hot spots if not used with proper stirring.
Water and oil baths involve placing the reaction vessel in a container of liquid heated on a hot plate. This method provides exceptionally uniform temperature distribution but is limited by the liquid's boiling point and potential fire hazards (with oil).
Sand baths offer a safer alternative to oil baths for higher temperatures. A container of sand is heated on a hot plate, providing good thermal stability, although with slower heat transfer.
For Cooling Reactions
Ice/water baths are the simplest method for maintaining a temperature of 0°C (32°F). They provide a large thermal mass that can absorb a significant amount of heat from an exothermic reaction.
Dry ice/solvent baths are used to achieve stable, sub-zero temperatures. The most common is a slurry of dry ice and acetone or isopropyl alcohol, which equilibrates at -78°C (-108°F).
Cryostats and chillers are automated, closed-loop systems. A pump circulates a refrigerated fluid through a coil immersed in the reaction or through a jacket surrounding the vessel. These offer the highest precision and control but are the most expensive option.
Understanding the Trade-offs
Choosing a temperature control method involves balancing precision, safety, and cost. There is no single "best" solution for all scenarios.
Precision vs. Cost
An ice bath is inexpensive and reliable for 0°C, but it offers no flexibility. A programmable circulating cryostat can maintain any temperature to within a tenth of a degree, but it represents a significant capital investment.
Your required precision dictates your cost. For a simple synthesis, a basic bath is often sufficient. For sensitive kinetic studies, an automated system is essential.
Safety Considerations
High-temperature oil baths carry a significant fire risk if the oil is heated past its flash point or spills onto the hot surface. Cryogenic liquids like dry ice and liquid nitrogen can cause severe burns and pose an asphyxiation hazard in poorly ventilated spaces.
Always evaluate the safety risks of your chosen method and implement appropriate engineering controls and personal protective equipment.
Scalability: From Lab to Plant
A method that works for a 100 mL flask may not be suitable for a 100-liter reactor. Immersing large vessels in baths is impractical and unsafe.
In industrial settings, jacketed reactors are standard. These vessels are surrounded by an outer shell (a "jacket") through which a thermal fluid is pumped from a large temperature control unit, allowing for precise and safe management of heat on a large scale.
Choosing the Right Method for Your Reaction
Select your approach based on the specific demands of your chemical process.
- If your primary focus is simplicity at a fixed temperature: Use a water/ice bath for 0°C or a specific dry ice/solvent slurry for lower fixed temperatures.
- If your primary focus is stable heating below 100°C: Use a stirred water bath for excellent thermal uniformity.
- If your primary focus is high precision and automation: Use a circulating chiller or cryostat connected to a jacketed vessel or immersion coil.
- If your primary focus is managing a powerful exothermic reaction: Use a cooling bath with a large thermal capacity (like an ice bath) and ensure the rate of addition of reagents is slow enough to not overwhelm the cooling system.
Mastering temperature control is fundamental to achieving a safe, efficient, and reproducible chemical synthesis.
Summary Table:
| Method | Best For | Key Considerations |
|---|---|---|
| Ice/Water Bath | Maintaining 0°C; simple, low-cost cooling | Large thermal mass; limited to specific temperature |
| Dry Ice/Solvent Bath | Achieving stable sub-zero temperatures (e.g., -78°C) | Requires handling of cryogenic materials |
| Heating/Oil Bath | Stable heating below the liquid's boiling point | Risk of hot spots and fire hazard with oil |
| Heating Mantle | Efficient, direct heating for round-bottom flasks | Requires vigorous stirring to avoid hot spots |
| Circulating Chiller/Cryostat | High-precision, automated control for any temperature | Highest cost; essential for sensitive or scalable reactions |
Achieve precise and safe temperature control in your lab.
Whether you are developing a new synthesis or scaling up a process, the right equipment is critical for managing exothermic heat and ensuring reaction reproducibility. KINTEK specializes in the lab equipment and consumables you need, from reliable heating mantles and baths to high-precision circulating chillers for jacketed reactors.
Let our experts help you select the ideal system for your specific temperature, safety, and scalability requirements.
Contact KINTEK today to optimize your chemical reactions.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Electric Split Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
People Also Ask
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?



















