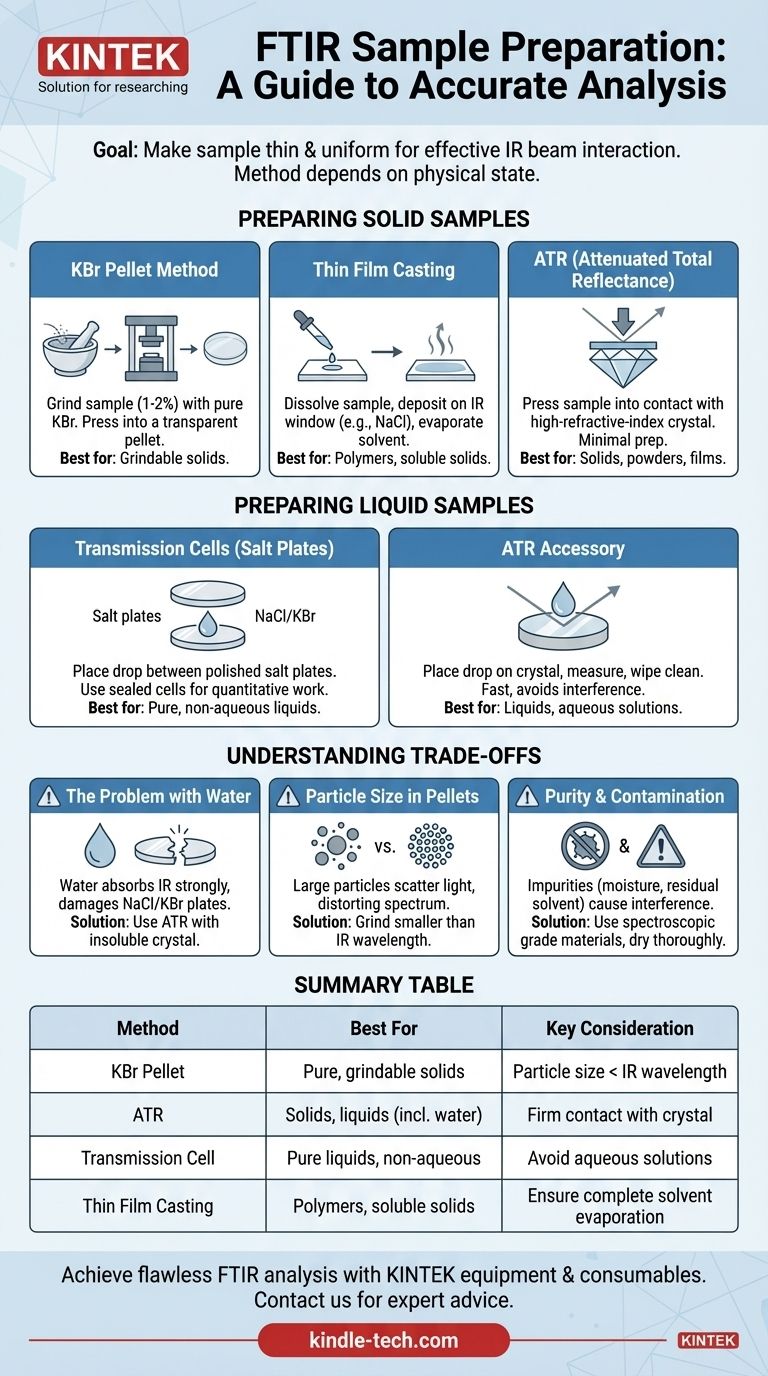
The fundamental goal of FTIR sample preparation is to make the sample sufficiently thin and uniform for the instrument's infrared beam to pass through it or interact with it effectively. While there are several methods, they all revolve around this principle, with the most common techniques for solids being the creation of pressed pellets and for liquids, the use of transmission cells or modern ATR accessories.
The optimal sample preparation method is not universal; it is dictated entirely by the physical state of your sample (solid, liquid, or gas) and your analytical objective. Choosing the right technique is the first and most critical step to acquiring a clean, interpretable spectrum.

Preparing Solid Samples
For a solid material to be analyzed by traditional transmission FTIR, it must be made nearly transparent to infrared radiation. This is typically achieved by dispersing the solid in an IR-transparent matrix or analyzing its surface directly.
The KBr Pellet Method
This is the classic technique for obtaining high-quality spectra from solid samples that can be ground into a powder. The sample is mixed with an alkali halide powder, most commonly potassium bromide (KBr).
The process involves grinding a small amount of the sample (1-2% by weight) with pure, dry KBr into an extremely fine powder. This mixture is then pressed under high pressure in a die to form a small, transparent, glass-like pellet.
The key to this method is that KBr is transparent to mid-infrared radiation and becomes viscous under pressure, allowing it to form a solid matrix that holds the finely dispersed sample particles.
Thin Film Casting
This method is ideal for polymers or other solids that are soluble in a volatile solvent. The solid is dissolved, and a few drops of the resulting solution are placed onto an IR-transparent window, such as a KBr or NaCl salt plate.
As the solvent evaporates, it leaves behind a thin, uniform film of the solid sample on the window, which can then be placed directly in the spectrometer's sample holder.
Attenuated Total Reflectance (ATR)
ATR is a modern, popular, and powerful technique that requires minimal to no sample preparation. It is a surface measurement technique, making it ideal for a wide range of solids, including powders, films, plastics, and even soft materials.
The sample is simply pressed into firm contact with a high-refractive-index crystal, often made of diamond, zinc selenide (ZnSe), or germanium (Ge). The IR beam is directed through the crystal in such a way that it interacts with the surface of the sample, generating a spectrum.
Preparing Liquid Samples
Analyzing liquids involves creating a thin, contained layer of the sample with a consistent thickness (pathlength) for the IR beam to pass through.
Transmission Cells (Salt Plates)
The most straightforward method for liquids is to place a single drop of the sample between two polished salt plates (typically NaCl or KBr).
Capillary action holds the plates together, and the liquid spreads to form a thin film. The plates are then placed in a holder and analyzed. For more precise quantitative work, sealed cells with a known pathlength are used instead of simple plates.
Using an ATR Accessory
Just as with solids, ATR is exceptionally useful for liquid analysis. A single drop of the liquid is placed onto the ATR crystal, the measurement is taken, and the crystal is then simply wiped clean.
This method is incredibly fast and avoids the potential for interference fringes that can occur with transmission cells. It is also the go-to method for analyzing aqueous solutions.
Understanding the Trade-offs
Choosing a method involves balancing ease of use with potential sources of error. An awareness of these pitfalls is critical for accurate analysis.
The Problem with Water
Water has intense, broad absorption bands in the infrared spectrum that can easily overwhelm the signal from your sample. Furthermore, the most common and inexpensive salt plates (NaCl and KBr) are soluble in water and will be damaged by aqueous solutions.
For samples containing water, ATR is the required method, using a water-insoluble crystal like diamond or ZnSe.
Particle Size in Pellets
When making KBr pellets, it is essential that the sample be ground into particles that are smaller than the wavelength of the infrared light being used.
If the particles are too large, they will scatter the light, causing a distorted, sloping baseline in the spectrum known as the Christiansen effect. This can obscure weaker peaks and make the spectrum difficult to interpret.
Purity and Contamination
Any substance that absorbs infrared radiation can be a contaminant. The KBr used for pellets must be spectroscopic grade and kept perfectly dry, as absorbed moisture will show up in the spectrum.
Likewise, when casting thin films, the solvent must be fully evaporated. Any residual solvent will contribute its own peaks to the final spectrum, confusing the analysis of your sample.
Making the Right Choice for Your Goal
Your choice of preparation method should be a deliberate decision based on your sample's properties and what you need to learn from it.
- If your primary focus is speed and simplicity for solids or non-aqueous liquids: ATR is the superior choice, as it requires virtually no sample preparation.
- If your primary focus is the highest quality spectrum for a pure, grindable solid: The KBr pellet method, when performed with care, provides excellent results for detailed analysis.
- If your primary focus is analyzing a pure liquid or a sample in a non-aqueous solvent: A simple transmission cell (salt plates) is effective and inexpensive.
- If your primary focus is analyzing any sample containing water: You must use an ATR accessory with a water-insoluble crystal.
Ultimately, the best preparation technique is the one that minimizes interference while accurately representing the chemical structure of your sample.
Summary Table:
| Method | Best For | Key Consideration |
|---|---|---|
| KBr Pellet | Pure, grindable solids | Particle size must be smaller than IR wavelength |
| ATR | Solids, liquids (including water), fast analysis | Requires firm contact with crystal (diamond, ZnSe) |
| Transmission Cell | Pure liquids, non-aqueous solutions | Avoid aqueous solutions to prevent salt plate damage |
| Thin Film Casting | Polymers, soluble solids | Ensure complete solvent evaporation |
Achieve flawless FTIR analysis with the right equipment from KINTEK.
Proper sample preparation is the foundation of accurate results. Whether you need durable ATR accessories with diamond crystals for aqueous samples, high-quality KBr for pellet pressing, or reliable transmission cells, KINTEK provides the precision lab equipment and consumables you need.
Our experts can help you select the ideal tools for your specific application, ensuring your laboratory operates at peak efficiency and accuracy.
Contact KINTEK today to discuss your FTIR needs and elevate your analytical capabilities!
Visual Guide
Related Products
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- How is a hydraulic press helpful for making KBr pellets? Achieve Superior FTIR Sample Preparation
- What is an example of a hydraulic press? Discover the Power of Laboratory Sample Preparation
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- What role does a laboratory hydraulic press play in pellet-type electrode fabrication? Enhancing Solid-State Performance
- Why is a laboratory hydraulic press essential for Ca3Co4O9 pelletizing? Optimize Pre-Sintering Mass Transport



















