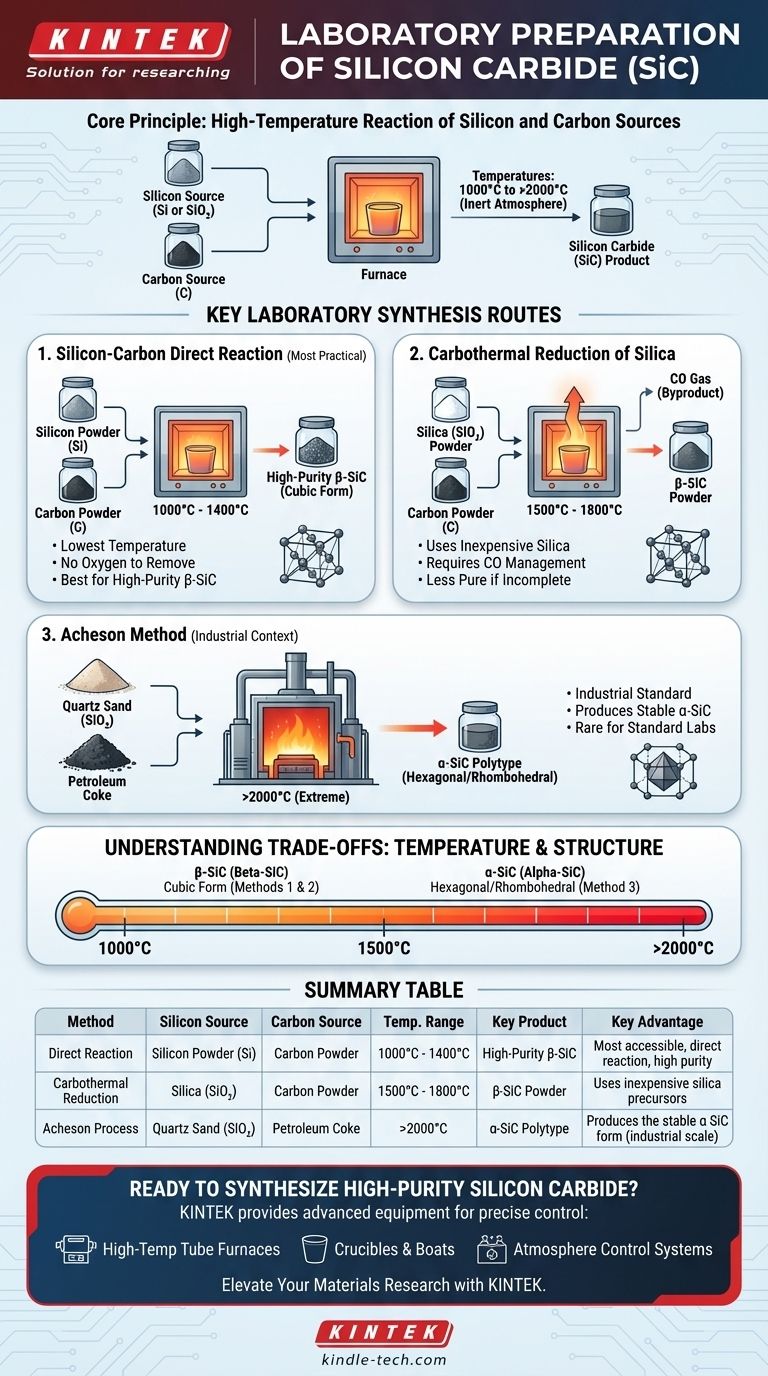
In the laboratory, silicon carbide (SiC) is typically prepared through one of three high-temperature powder synthesis methods. These involve reacting a silicon source (elemental silicon or silicon dioxide) with a carbon source at temperatures ranging from 1000°C to over 2000°C in a controlled furnace environment.
The most practical method for many laboratories is the direct reaction of silicon and carbon powders, as it requires the lowest temperature and can produce high-purity β-SiC. Your choice of method ultimately depends on your available equipment and the specific type of SiC you need to synthesize.

Understanding the Core Chemistry
All synthesis routes for silicon carbide are built on the same fundamental principle: creating a chemical environment where silicon and carbon atoms bond covalently at high temperatures. The specific sources of these elements and the temperature used dictate the final product's characteristics.
The Silicon Source: Silicon vs. Silica
The initial form of silicon is a critical decision point. You can start with either high-purity elemental silicon powder (Si) or silicon dioxide (SiO₂) powder, often called silica. Using pure silicon leads to a more direct reaction, while using silica involves a reduction step.
The Carbon Source: Purity is Paramount
The carbon source is typically a fine powder like petroleum coke, carbon black, or graphite. The purity of the carbon source directly impacts the purity of the resulting SiC, so using high-purity materials is essential for high-quality synthesis.
Key Laboratory Synthesis Routes
While industrial methods operate on a massive scale, their underlying chemistry is directly applicable to laboratory synthesis. The three primary routes offer different trade-offs in temperature, purity, and complexity.
Method 1: Silicon-Carbon Direct Reaction
This is often the most accessible method for a well-equipped materials lab. It involves heating an intimate mixture of high-purity silicon powder and carbon powder.
The reaction is straightforward: Si + C → β-SiC.
This process is typically run at temperatures between 1000°C and 1400°C. Its main advantage is producing high-purity β-SiC because there are no other elements, like oxygen from silica, to remove.
Method 2: Carbothermal Reduction of Silica
This common method uses inexpensive silica powder as the silicon source. It is mixed with carbon powder and heated to a higher temperature range.
The reaction is: SiO₂ + 3C → β-SiC + 2CO (gas).
This requires temperatures between 1500°C and 1800°C. It successfully produces β-SiC powder, but requires careful management of the carbon monoxide (CO) gas byproduct and may result in a less pure product if the reaction is incomplete.
Method 3: The Acheson Method (Industrial Context)
The Acheson method is the primary industrial process for producing SiC. It involves heating a massive mixture of quartz sand (SiO₂) and petroleum coke to extreme temperatures.
This process operates above 2000°C and is the standard way to synthesize the hard, stable α-SiC polytype. Due to the extreme energy and equipment requirements, this method is rarely replicated on a standard laboratory scale.
Understanding the Trade-offs
Choosing the right synthesis route requires balancing three key factors: the desired crystal structure, required purity, and your laboratory's capabilities.
Temperature Determines the Crystal Structure (Polytype)
The most significant factor is temperature. The crystal structure, or polytype, of SiC is a direct result of the synthesis temperature.
- β-SiC (Beta-SiC): This cubic form is synthesized at lower temperatures, typically below 2000°C. Both the direct reaction and carbothermal reduction methods produce β-SiC.
- α-SiC (Alpha-SiC): These hexagonal and rhombohedral forms are more thermodynamically stable and are synthesized at very high temperatures, generally above 2000°C, via the Acheson process.
Precursors Define Final Purity
The purity of your final SiC powder is limited by the purity of your starting materials. The direct reaction of silicon and carbon generally offers a cleaner route to a high-purity product.
Equipment and Atmosphere Control are Crucial
All these methods require a high-temperature furnace capable of reaching at least 1400°C. The process must be run in an inert atmosphere (like argon) to prevent the silicon and carbon from oxidizing, which would ruin the synthesis.
Selecting the Right Method for Your Goal
Your choice should be guided by your specific experimental objectives and lab constraints.
- If your primary focus is high-purity β-SiC with accessible equipment: The direct reaction of silicon and carbon powders is the most straightforward and controllable approach.
- If you are working with silica precursors and have a high-temperature furnace: The carbothermal reduction method is a viable and classic route to produce β-SiC powder.
- If your goal is to produce the α-SiC polytype: You will need specialized, high-temperature equipment capable of reaching temperatures well above 2000°C, mirroring an industrial process.
Ultimately, successful laboratory synthesis of silicon carbide hinges on matching your precursor materials and temperature capabilities to the specific SiC properties you aim to achieve.
Summary Table:
| Method | Silicon Source | Carbon Source | Temperature Range | Key Product | Key Advantage |
|---|---|---|---|---|---|
| Direct Reaction | Silicon Powder (Si) | Carbon Powder | 1000°C - 1400°C | High-Purity β-SiC | Most accessible, direct reaction, high purity |
| Carbothermal Reduction | Silica (SiO₂) | Carbon Powder | 1500°C - 1800°C | β-SiC Powder | Uses inexpensive silica precursors |
| Acheson Process | Quartz Sand (SiO₂) | Petroleum Coke | >2000°C | α-SiC Polytype | Produces the stable α-SiC form (industrial scale) |
Ready to Synthesize High-Purity Silicon Carbide in Your Lab?
Choosing the right synthesis method is just the first step. Achieving consistent, high-quality results requires precise temperature control and a reliable inert atmosphere—exactly what KINTEK's advanced lab furnaces provide.
KINTEK specializes in the high-temperature equipment and consumables you need for advanced materials synthesis, including:
- High-Temperature Tube Furnaces: Precisely control temperatures up to 1800°C and beyond in an inert atmosphere.
- Crucibles & Boats: High-purity alumina or graphite containers designed for SiC synthesis reactions.
- Atmosphere Control Systems: Ensure your reactions are protected from oxidation.
Let our experts help you select the perfect setup for your specific SiC synthesis goals, whether you're targeting β-SiC or the more challenging α-SiC polytype.
Contact KINTEK today to discuss your laboratory's needs and elevate your materials research!
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
People Also Ask
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies
- What is the range of pyrolysis? Master Temperature Control for Optimal Bio-Product Yields
- What are the equipment requirements for loading platinum (Pt) onto composite supports? Precise Stirring for High Dispersion
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process



















