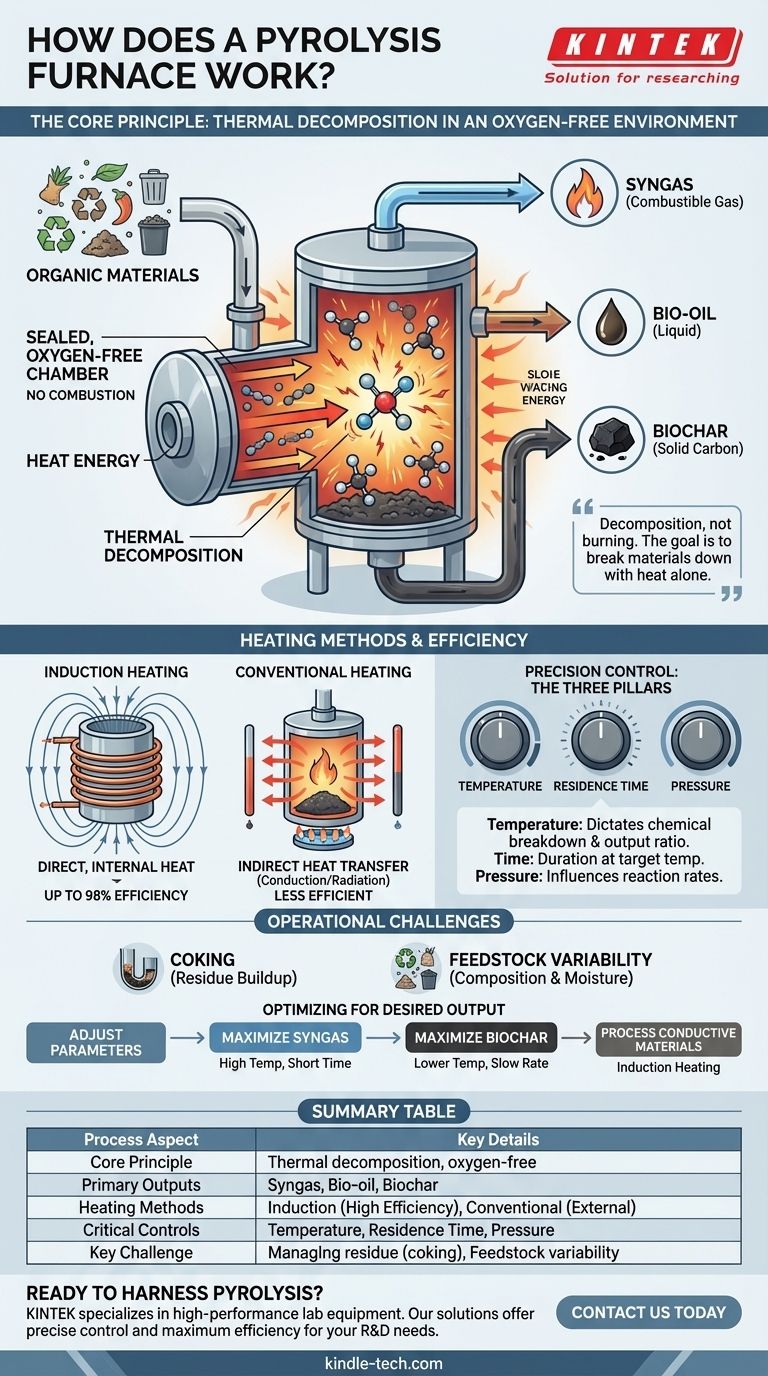
At its core, a pyrolysis furnace is a specialized, high-temperature reactor. It works by heating organic materials in a sealed, oxygen-free chamber. This absence of oxygen is critical; it ensures the material thermally decomposes—breaking down its chemical bonds—rather than burning. The process transforms complex organic waste into simpler, often valuable, products like gas, liquid oils, and a solid carbon-rich residue called char.
A pyrolysis furnace is not defined by a single heating method, but by its purpose: to create a precisely controlled, oxygen-starved environment. The true goal is to break materials down with heat alone, making the careful management of temperature, time, and pressure the most critical factors for success.

The Fundamental Principle: Decomposition Without Combustion
The entire operation of a pyrolysis furnace hinges on one foundational concept: preventing combustion while applying intense heat. This allows for a complete transformation of the material's chemical structure.
The Critical Role of an Oxygen-Free Environment
In a normal fire, oxygen acts as an oxidizer, causing the material to combust and release energy as heat and light, leaving behind ash. By removing virtually all oxygen, a pyrolysis furnace prevents this reaction. Instead of burning, the material is forced to decompose under thermal stress.
How Thermal Decomposition Works
Heat is a form of energy. When applied to the feedstock inside the furnace, this energy causes the molecules to vibrate violently until their chemical bonds break. Large, complex organic molecules are systematically broken down into smaller, more stable molecules.
The Three Primary Outputs
The specific outputs depend on the feedstock and process conditions, but they generally fall into three categories:
- Syngas: A mixture of combustible gases like hydrogen, methane, and carbon monoxide.
- Bio-oil/Pyrolysis Oil: A liquid tar-like substance that can be refined into other chemicals or fuels.
- Biochar/Coke: A stable, solid material rich in carbon.
How Heat is Generated and Controlled
While the principle is universal, the method for generating and applying heat can vary. The choice of heating technology often depends on the material being processed and the desired efficiency.
Induction Heating: Direct and Efficient
A common and highly effective method is induction heating. A high-frequency alternating current is passed through a copper coil wrapped around the chamber. This creates a powerful, fluctuating magnetic field.
If the material inside (the "charge") is electrically conductive, this magnetic field induces internal electric currents called eddy currents. The material's own electrical resistance to these currents generates intense, rapid heat directly within the feedstock itself. This is highly efficient, with some core-type induction furnaces achieving up to 98% power efficiency.
Conventional Heating Systems
Other furnaces rely on more traditional external heating elements or gas burners. In these systems, heat is generated outside the primary chamber and transferred to the material through thermal conduction and radiation. While effective, this can be less efficient than the direct heating provided by induction.
The Three Pillars of Control
Regardless of the heat source, successful pyrolysis depends on precise control over three key parameters:
- Temperature: Determines which chemical bonds break and influences the ratio of gas, liquid, and solid outputs.
- Residence Time: The duration the material is held at the target temperature, impacting the completeness of the decomposition.
- Pressure: The internal pressure of the furnace can influence reaction rates and the final composition of the products.
Understanding Operational Challenges
Operating a pyrolysis furnace involves more than just heat and chemistry. Real-world implementation requires managing byproducts, efficiency, and the feedstock itself.
Managing Residue and "Coking"
The solid char produced can sometimes build up on the internal surfaces of the furnace, a process known as coking. This residue can insulate the chamber, reduce efficiency, and require periodic removal through automated or manual "decoking" procedures.
The Importance of Feedstock
The furnace's performance is directly tied to the material it processes. The composition, moisture content, and density of the organic waste all affect how it will behave under heat and what the final products will be.
Efficiency vs. Throughput
There is often a trade-off between the speed of the process and the quality of the outputs. A longer residence time may produce a more desirable product but reduces the overall throughput of the system. Finding the optimal balance is key to economic viability.
Making the Right Choice for Your Goal
The ideal operation of a pyrolysis furnace is dictated entirely by the desired end product. By adjusting the core parameters, you can steer the chemical reactions toward a specific outcome.
- If your primary focus is maximizing syngas production: You will typically use very high temperatures and shorter residence times to favor the complete breakdown of materials into gaseous components.
- If your primary focus is producing high-quality biochar: You will generally use lower temperatures and a slower heating rate to preserve the carbon structure in a solid form.
- If your primary focus is processing conductive metals or waste: An induction furnace is the ideal choice, as its heating mechanism is uniquely suited to directly and efficiently heat these materials.
Ultimately, mastering a pyrolysis furnace is about leveraging precise control over its internal environment to dictate the exact outcome of thermal decomposition.
Summary Table:
| Process Aspect | Key Details |
|---|---|
| Core Principle | Thermal decomposition in an oxygen-free environment (no combustion). |
| Primary Outputs | Syngas, Bio-oil/Pyrolysis Oil, Biochar/Coke. |
| Heating Methods | Induction Heating (highly efficient) or Conventional Heating (external elements/burners). |
| Critical Controls | Temperature, Residence Time, Pressure. |
| Key Challenge | Managing residue buildup (coking) and feedstock variability. |
Ready to harness the power of pyrolysis in your lab?
KINTEK specializes in high-performance lab equipment, including advanced pyrolysis systems. Whether your goal is efficient waste processing, material synthesis, or R&D, our solutions offer precise temperature control and maximum efficiency to achieve your desired outputs—be it syngas, bio-oil, or high-quality biochar.
Contact us today to discuss how our pyrolysis furnaces can be tailored to your specific laboratory needs.
Get in touch via our Contact Form
Visual Guide
Related Products
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- Mesh belt controlled atmosphere furnace
- Laboratory High Throughput Tissue Grinding Mill Grinder
- Laboratory Hybrid Tissue Grinding Mill
- Engineering Advanced Fine Ceramics Aluminum Oxide Al2O3 Heat Sink for Insulation
People Also Ask
- What role does a high-vacuum high-temperature furnace serve in nuclear-grade Uranium Carbide simulations?
- Why is a high-precision industrial electric furnace required for metal normalizing? Unlock Superior Grain Refinement
- What is the process temperature of an electric arc furnace? Harnessing Extreme Heat for Steelmaking
- What are the benefits of metal sintering? Achieve Complex, Cost-Effective Metal Parts
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs
- What are the advantages of using a laboratory vacuum drying oven for SRB regeneration? Preserve Biological Viability
- What does heat treatment do to microstructure properties? Tailor Material Strength, Hardness, and Toughness
- How do you test for a leak in a vacuum furnace? Ensure Process Purity and Prevent Contamination



















