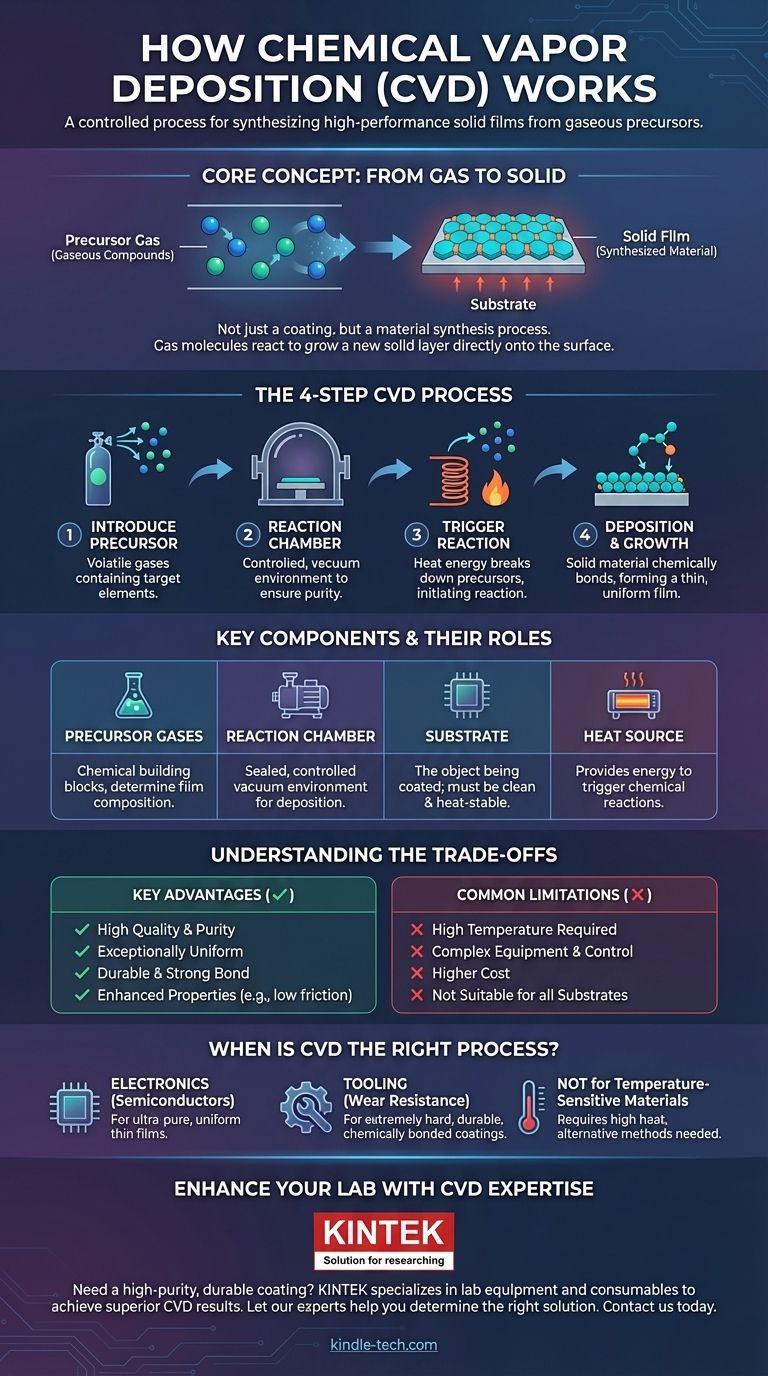
At its core, Chemical Vapor Deposition (CVD) is a process for creating a high-performance solid film on a surface. It works by introducing a precursor gas into a chamber, which then undergoes a chemical reaction triggered by heat. This reaction causes a solid material to form and bond directly onto the surface of a target object, or substrate, building up a thin, uniform coating.
The crucial concept to grasp is that CVD is not simply a coating method; it's a material synthesis process. You are not "spraying" a pre-existing substance, but rather using controlled chemical reactions in a gaseous state to grow a new, solid layer directly onto a component's surface.

The Core Principle: From Gas to Solid
The entire CVD process is built on a controlled transformation of matter. A carefully chosen gas is converted into a solid thin film through a precise sequence of events inside a reaction chamber.
Introducing the Precursor
The process begins with one or more volatile precursor gases. These are gaseous chemical compounds that contain the specific elements you want to deposit.
The Reaction Chamber
The object to be coated, known as the substrate, is placed inside a sealed chamber. This chamber is typically put under a vacuum to remove any air or contaminants that could interfere with the chemical reaction.
Triggering the Reaction
The substrate is heated to a specific reaction temperature. This applied energy breaks down the precursor gases, causing them to react either with each other or with the substrate itself.
Deposition and Film Growth
The product of this chemical reaction is the desired solid material. This new material deposits onto the heated substrate, molecule by molecule, forming a strong chemical bond with the surface and gradually building up a thin, even film.
A Closer Look at the Key Components
Understanding the role of each component clarifies how CVD achieves such precise results. Each element is critical for controlling the outcome of the final film.
The Substrate
The substrate is the workpiece or component that receives the coating. Its surface must be meticulously clean, and its ability to withstand high temperatures is a key factor in the process.
The Precursor Gases
These are the building blocks of the new film. The selection of precursors is critical, as their chemical makeup directly determines the composition of the final coating, whether it's silicon nitride, titanium carbide, or another material.
The Vacuum Environment
The vacuum serves two purposes. First, it ensures process purity by removing unwanted particles. Second, it allows for better control over the movement and concentration of the precursor gases as they flow toward the substrate.
The Chemical Transport Method
In some variations of CVD, the process is slightly different. A solid or liquid substance first reacts in a "source area" to become a gas. This gas is then transported to the substrate (the "growth area"), where a reverse chemical reaction causes it to deposit back into its solid form.
Understanding the Trade-offs
Like any advanced manufacturing process, CVD involves a balance of powerful benefits and practical limitations. Understanding these trade-offs is key to determining its suitability for a given application.
Key Advantages
The primary benefit of CVD is the quality of the film. Because it's grown chemically, the coating is often highly pure, dense, and exceptionally uniform, even over complex shapes. This results in durable surfaces with enhanced properties, such as decreased friction or increased thermal resistance.
Common Limitations
The main drawback is the high temperature required for many CVD reactions. This can damage or alter substrates that are not thermally stable. The process also requires complex equipment and precise control, making it more expensive than simpler coating methods like painting or electroplating.
When is CVD the Right Process?
Choosing CVD depends entirely on your end goal. The process excels where performance and purity are paramount, but may be overkill for less demanding applications.
- If your primary focus is creating ultra-pure, uniform thin films for electronics: CVD is the industry standard for manufacturing semiconductors and integrated circuits due to its unmatched precision.
- If your primary focus is enhancing the surface properties of a tool or component: CVD is ideal for creating extremely hard, wear-resistant, or corrosion-resistant coatings that are chemically bonded to the substrate.
- If your primary focus is coating a temperature-sensitive material: Traditional high-temperature CVD is unsuitable, and you must explore lower-temperature alternatives or entirely different deposition techniques.
Ultimately, Chemical Vapor Deposition provides a powerful method for engineering material surfaces at the molecular level.
Summary Table:
| Key Component | Role in the CVD Process |
|---|---|
| Precursor Gases | The chemical building blocks that react to form the solid film. |
| Reaction Chamber | A sealed, controlled environment (often under vacuum) where the deposition occurs. |
| Substrate | The object being coated; its surface must be clean and thermally stable. |
| Heat Source | Provides the energy to trigger the chemical reaction that deposits the solid material. |
Need a high-purity, durable coating for your lab components or production tools?
The precise control of Chemical Vapor Deposition is key to creating films that enhance wear resistance, thermal stability, and performance. At KINTEK, we specialize in providing the lab equipment and consumables needed to achieve these superior results.
Let our experts help you determine if CVD is the right solution for your application. Contact us today to discuss your specific laboratory needs and how our solutions can bring value to your work.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the composition of a CVD diamond? Discover the Pure Carbon Structure of Lab-Grown Gems
- What is the Chemical Vapour Deposition (CVD) method for growing diamonds? Discover Precision Carbon Synthesis
- What are the parts of chemical vapor deposition? A Guide to CVD System Components
- What is pyrolysis in the context of single-layer graphene manufacturing? Overcoming the 1000°C Thermal Barrier
- What is the difference between CVD and MOCVD? Precision vs. Versatility in Thin-Film Deposition
- What is the pressure in LPCVD? Master the Key to Superior Film Uniformity
- What are the two fundamental steps in the creation of CVD graphene? Master Pyrolysis and Structure Formation
- What is the primary function of a High Vacuum CVD Furnace? Master High-Quality Graphene Synthesis



















