In essence, Chemical Vapor Deposition (CVD) grows a diamond by breaking down carbon-rich gases in a vacuum and depositing the carbon atoms, layer by layer, onto a diamond seed crystal. This process doesn't rely on immense pressure like natural diamond formation but instead uses high energy and specific chemical reactions to build a diamond's crystalline structure from the bottom up.
The core principle of CVD diamond creation is not about compressing carbon, but about carefully arranging individual carbon atoms from a gas onto a pre-existing diamond template. It is a method of controlled, atomic-level construction rather than a simulation of raw geological force.

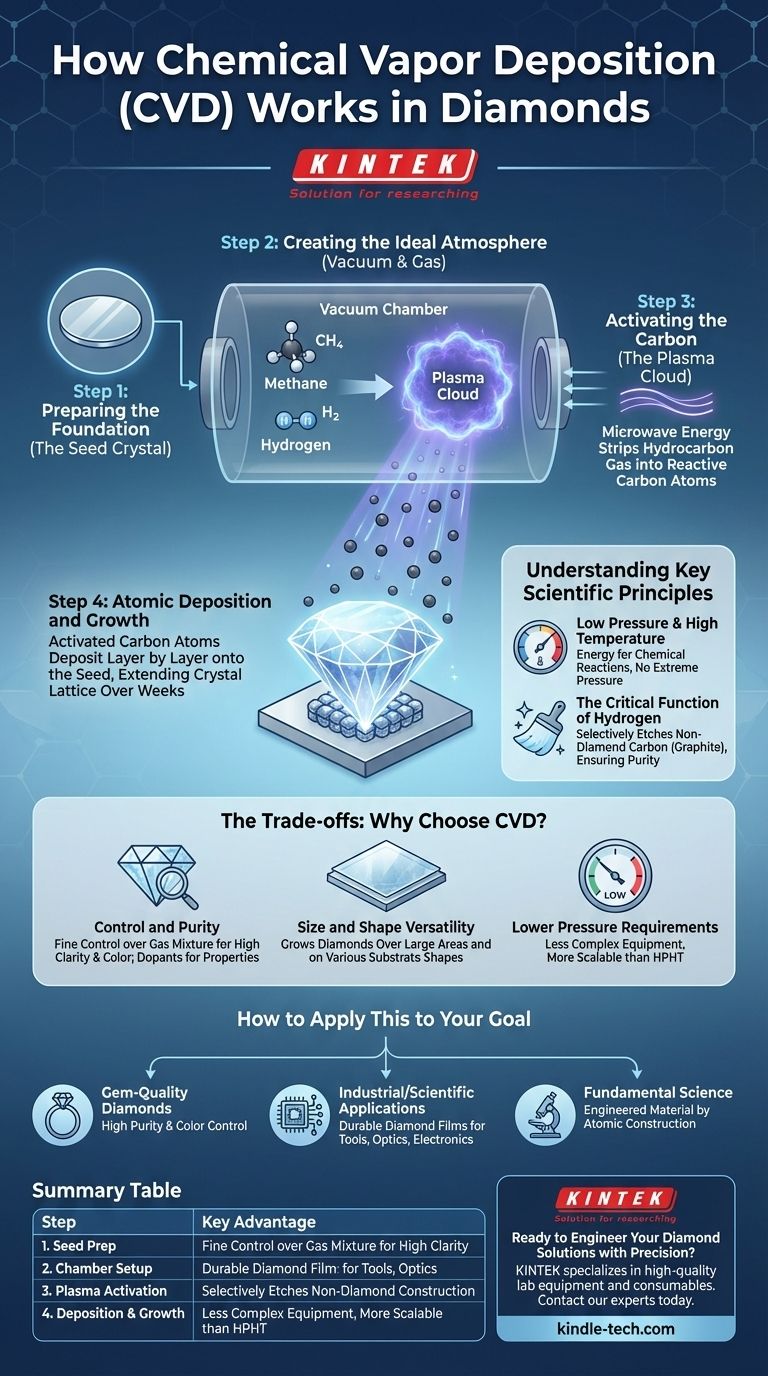
The CVD Diamond Growth Process: A Step-by-Step Breakdown
To understand how a gas transforms into one of the hardest materials on Earth, we must look at the process in distinct phases. Each step is precisely controlled within a specialized reactor chamber.
Step 1: Preparing the Foundation (The Seed Crystal)
The process begins with a substrate, which is typically a very thin slice of a pre-existing diamond, known as a seed crystal. This seed provides the atomic blueprint, ensuring the new carbon atoms arrange themselves into the correct diamond lattice structure.
Step 2: Creating the Ideal Atmosphere (Vacuum & Gas)
The diamond seed is placed inside a vacuum chamber. All air is removed, and a carefully controlled mixture of gases is introduced. The primary ingredient is a hydrocarbon gas, such as methane (CH₄), which serves as the source of carbon.
Step 3: Activating the Carbon (The Plasma Cloud)
Energy, usually in the form of microwaves, is introduced into the chamber. This intense energy strips the hydrocarbon gas molecules apart, creating a glowing cloud of chemically reactive particles called a plasma.
This plasma contains free carbon atoms along with other atomic fragments. The temperature within this plasma can be exceptionally high, creating the perfect environment for the necessary chemical reactions to occur.
Step 4: Atomic Deposition and Growth
The activated carbon atoms from the plasma are drawn down and settle onto the surface of the cooler diamond seed. Following the template provided by the seed, these atoms bond to the surface, extending the crystal lattice.
This happens layer by atomic layer, slowly growing the diamond over a period of weeks. The result is a larger, fully formed diamond that is chemically and structurally identical to the seed it grew from.
Understanding the Key Scientific Principles
The success of CVD hinges on managing a delicate balance of conditions that favor diamond growth over the formation of other, less desirable forms of carbon like graphite.
The Role of Low Pressure and High Temperature
Unlike the High-Pressure, High-Temperature (HPHT) method that mimics the Earth's mantle, CVD operates at very low pressures. The high temperature of the plasma provides the energy needed for the chemical reactions, eliminating the need for crushing physical force.
The Critical Function of Hydrogen
The gas mixture isn't just carbon; it's also rich in hydrogen. Hydrogen plays a crucial role by selectively etching away any non-diamond carbon (graphite) that might try to form on the surface. This "cleans" the growth surface, ensuring only the strong diamond lattice structure can grow.
CVD vs. PVD: A Key Distinction
It's important not to confuse CVD with Physical Vapor Deposition (PVD). PVD involves physically heating a material until it vaporizes and then condenses on a target. In contrast, CVD is a chemical process where gases react on the substrate's surface to form the new material.
The Trade-offs: Why Choose CVD?
CVD is not just another way to make a diamond; it offers distinct advantages and trade-offs compared to other methods, making it uniquely suited for specific applications.
Control and Purity
The primary advantage of CVD is fine control. By precisely managing the gas mixture, operators can minimize impurities and create diamonds of exceptionally high purity and clarity. This also allows for the intentional introduction of elements to create specific colors or electronic properties.
Size and Shape Versatility
Because it is a deposition process, CVD can be used to grow diamonds over large areas and on various substrate shapes. This makes it ideal for creating diamond coatings on industrial tools, optics, and semiconductor components, a feat not possible with HPHT.
Lower Pressure Requirements
The lack of extreme pressure makes the equipment for CVD generally less complex and potentially more scalable than the massive presses required for HPHT synthesis.
How to Apply This to Your Goal
The CVD method's characteristics make it suitable for different objectives, from gem creation to advanced technological development.
- If your primary focus is creating large, high-purity gem-quality diamonds: CVD offers exceptional control over clarity and color by carefully managing the gas mixture during the growth process.
- If your primary focus is industrial or scientific applications: CVD's ability to coat large, complex surfaces makes it the superior method for creating durable diamond films for electronics, cutting tools, and high-performance windows.
- If your primary focus is understanding the fundamental science: CVD demonstrates that diamond is an engineered material that can be built atom-by-atom, defined by its crystal structure rather than its origin.
Ultimately, Chemical Vapor Deposition empowers us to engineer diamond for purposes and with a precision that far exceeds what natural geology can provide.
Summary Table:
| CVD Diamond Growth Steps | Key Process Details |
|---|---|
| 1. Seed Preparation | A thin diamond seed crystal provides the atomic template for growth. |
| 2. Chamber Setup | A vacuum chamber is filled with a carbon-rich gas mixture (e.g., methane). |
| 3. Plasma Activation | Microwaves create a high-energy plasma, breaking down the gas into reactive carbon atoms. |
| 4. Deposition & Growth | Carbon atoms deposit onto the seed, building the diamond lattice layer by layer over weeks. |
| Key Advantage | Fine control over purity, clarity, and the ability to coat large or complex shapes. |
Ready to Engineer Your Diamond Solutions with Precision?
The controlled, layer-by-layer process of Chemical Vapor Deposition (CVD) is key to creating high-purity diamonds for advanced applications. Whether your goal is producing flawless gemstones or developing cutting-edge industrial components, the right equipment is critical for success.
KINTEK specializes in high-quality lab equipment and consumables, serving the precise needs of laboratories focused on material science and synthesis. Let our expertise help you achieve unparalleled control and results in your diamond growth projects.
Contact our experts today to discuss how our solutions can power your innovation.
Visual Guide
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- CVD Diamond Domes for Industrial and Scientific Applications
- CVD Diamond Cutting Tool Blanks for Precision Machining
People Also Ask
- What is the purpose of adding a boron source in CVD diamond growth? Master P-Type Semiconductor Conductivity
- What is chemical vapor deposition in Nanomanufacturing? The Ultimate Guide to Atomic-Level Material Engineering
- Why do we use CVD? Unlock Unmatched Precision in Thin Film Deposition
- What are the CVD reactor types? Select the Right Process for Your Material and Substrate
- What is ion sputtering? A Guide to High-Precision Thin Film Deposition
- What is CVD in nanomaterials? A Guide to High-Purity Material Fabrication
- Which type of sputtering system is used to deposit ZnO thin film? Discover RF Magnetron Sputtering for Superior Films
- Why is ALD better than CVD? Precision vs. Speed in Thin Film Deposition



















