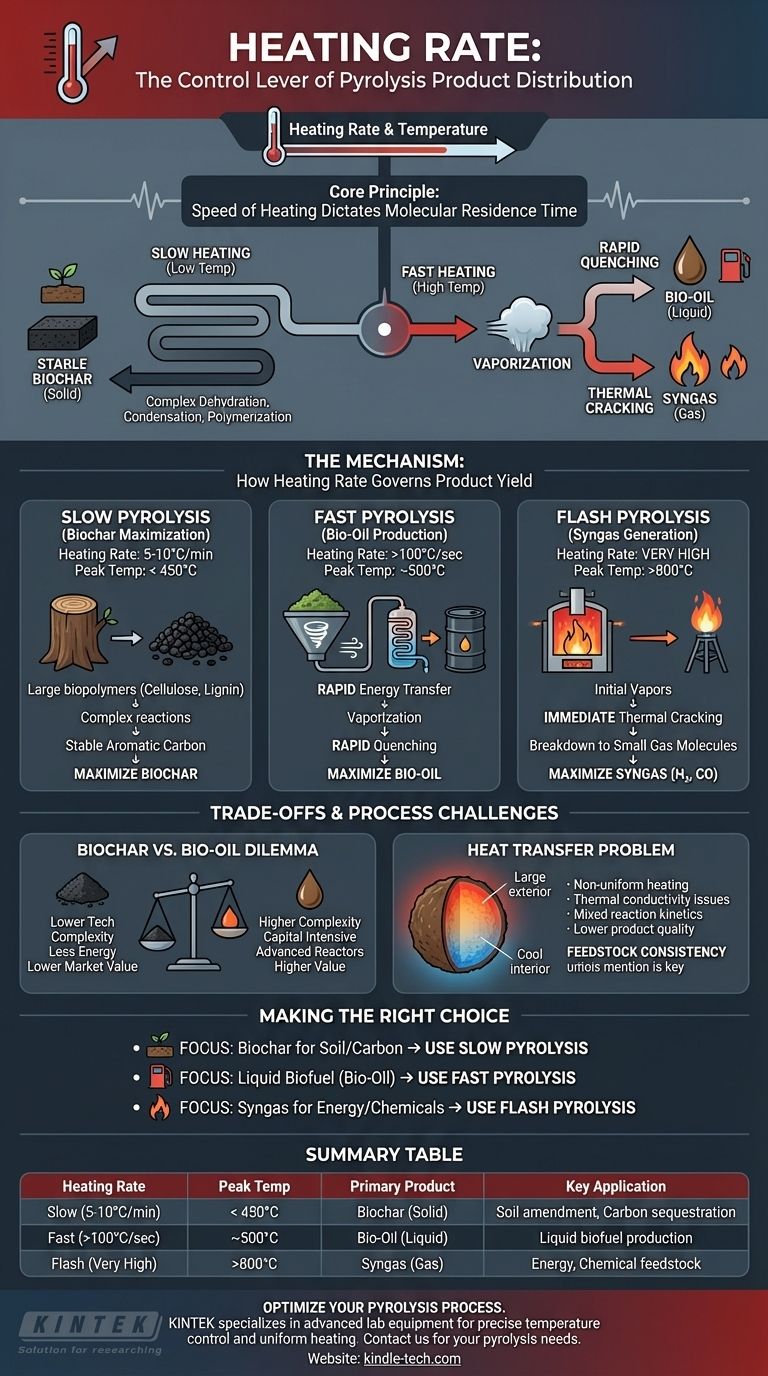
Heating rate is a primary control lever in pyrolysis, directly determining the final product distribution. Slow heating rates at lower temperatures favor the production of solid biochar, whereas rapid heating rates at higher temperatures favor the production of liquids (bio-oil) and gases. The speed at which you apply heat dictates which chemical decomposition pathways are prioritized.
The core principle is simple: the speed of heating determines the residence time of molecules at specific temperatures. Slow heating allows for reactions that create stable solids, while fast heating rapidly vaporizes material into vapors that can be condensed into liquid oil or further broken down into gas.

The Mechanism: How Heating Rate Governs Product Yield
Pyrolysis is the thermal decomposition of organic material in the absence of oxygen. The heating rate, alongside temperature, is the most critical parameter you can control to steer the outcome of this process. It fundamentally alters the reaction kinetics.
Slow Pyrolysis for Biochar Maximization
Slow heating rates (e.g., 5-10°C per minute) combined with relatively low peak temperatures (typically below 450°C) are the ideal conditions for producing biochar.
This process gives large biopolymer molecules (like cellulose and lignin) sufficient time to undergo complex dehydration, condensation, and polymerization reactions. This favors the formation of stable, cross-linked aromatic carbon structures, maximizing the solid char residue.
Fast Pyrolysis for Bio-Oil Production
Fast pyrolysis uses very high heating rates (often >100°C per second) and moderate temperatures (around 500°C). The goal is to maximize bio-oil, a liquid fuel.
This rapid energy transfer breaks down the biomass so quickly that it vaporizes before significant charring can occur. These hot vapors are then rapidly cooled, or "quenched," to condense them into a liquid mixture of hundreds of different organic compounds known as bio-oil.
Flash Pyrolysis and Gasification
At very high temperatures (above 800°C) and extremely rapid heating rates, the primary product becomes syngas (synthesis gas), a mixture of hydrogen (H₂) and carbon monoxide (CO).
Under these conditions, the initial pyrolysis vapors don't have time to condense. Instead, they are immediately thermally "cracked"—broken down into the smallest, most stable gas molecules. This process is often considered gasification rather than traditional pyrolysis.
Understanding the Trade-offs and Process Challenges
Choosing a heating rate is not just about the desired product; it's about balancing engineering complexity, energy input, and product quality.
The Biochar vs. Bio-oil Dilemma
Slow pyrolysis is technologically simpler and less energy-intensive to operate. However, its primary output, biochar, often has a lower market value than liquid fuels.
Fast pyrolysis systems are more complex and capital-intensive. They require sophisticated reactors to achieve rapid heat transfer and quenching systems to capture the bio-oil, presenting a greater engineering challenge.
The Problem of Heat Transfer
Achieving a uniform, high heating rate throughout a large volume of biomass is difficult. Biomass is a poor thermal conductor, meaning the surface can heat much faster than the core.
This temperature gradient can lead to a mixed reaction, where the outside of a particle undergoes fast pyrolysis while the inside undergoes slow pyrolysis. The result is a lower-quality, mixed product stream with lower yields of the target product.
Feedstock Consistency is Key
The ideal heating rate and temperature profile can vary depending on the feedstock's composition, particle size, and moisture content. What works perfectly for wood chips may be suboptimal for agricultural waste, requiring process adjustments to maintain efficiency.
Making the Right Choice for Your Goal
Your choice of heating rate must be a deliberate decision aligned with your primary objective.
- If your primary focus is producing biochar for soil amendment or carbon sequestration: Employ a slow pyrolysis process with controlled, low heating rates and peak temperatures below 450°C.
- If your primary focus is creating liquid biofuel (bio-oil): Implement a fast pyrolysis system with very high heating rates, moderate temperatures, and a rapid vapor quenching mechanism.
- If your primary focus is generating syngas for energy or chemical feedstock: Use a high-temperature, rapid heating process that ensures thermal cracking of all volatile components into non-condensable gases.
By mastering the heating rate, you transform pyrolysis from a simple decomposition reaction into a precise tool for targeted material production.
Summary Table:
| Heating Rate | Peak Temperature | Primary Product | Key Application |
|---|---|---|---|
| Slow (5-10°C/min) | < 450°C | Biochar (Solid) | Soil amendment, carbon sequestration |
| Fast (>100°C/sec) | ~500°C | Bio-Oil (Liquid) | Liquid biofuel production |
| Flash (Very High) | >800°C | Syngas (Gas) | Energy or chemical feedstock |
Ready to optimize your pyrolysis process for maximum yield and efficiency? KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Whether you're targeting biochar, bio-oil, or syngas production, our solutions ensure precise temperature control and uniform heating for reliable results. Contact our experts today to discuss how we can support your laboratory's pyrolysis needs!
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the types of pyrolysis reactors used in industry? Choose the Right Technology for Your Product
- What is the best catalyst for plastic pyrolysis? Match Your Catalyst to Your Plastic Waste Goals
- What is safety in pyrolysis process? Managing Extreme Heat and Flammable Products
- What is the role of a Rotary Furnace in recycling nickel-based superalloys? Unlocking Critical Metal Recovery
- What energy product is produced by pyrolysis? Biochar, Bio-oil, and Syngas Explained
- Why is pyrolysis sustainable? Unlocking a Circular Economy with Waste-to-Value Technology
- What are the results of calcination? A Guide to Purification and Material Transformation
- What is the biochar in pyrolysis reaction? Unlocking Its Role in Soil Enhancement and Carbon Sequestration



















