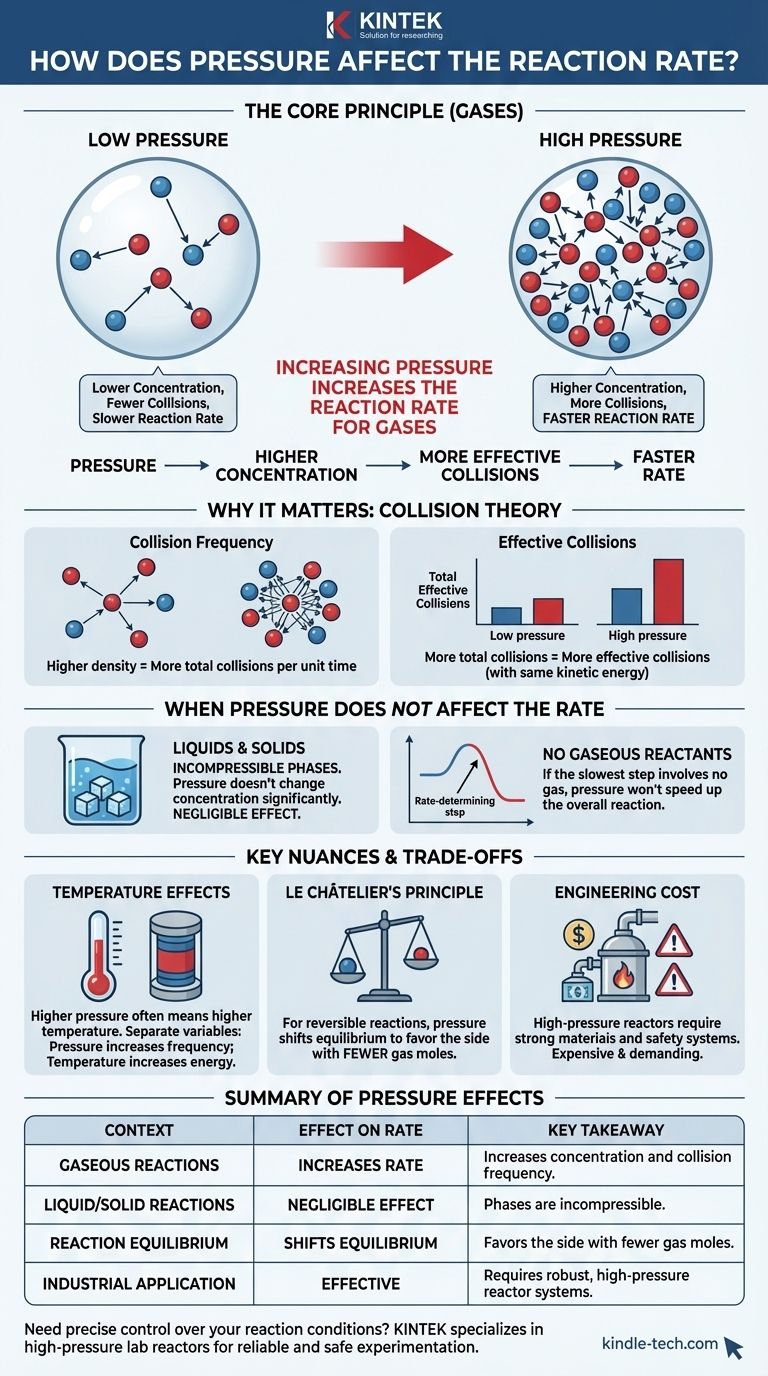
In short, increasing the pressure of a reaction involving gases increases the reaction rate. This occurs because pressure is a direct measure of concentration for gases. By compressing the gas molecules into a smaller volume, you increase the likelihood that they will collide and react with one another.
The core principle is that for gaseous reactions, pressure acts as a direct lever on concentration. Higher pressure forces gas molecules closer together, increasing the frequency of effective collisions and thereby accelerating the reaction. This effect is negligible for reactions in the liquid or solid phase.

The Fundamental Mechanism: Why Pressure Matters
To truly understand the effect of pressure, we need to go back to the first principles of how chemical reactions happen. It all revolves around the concept of particles colliding.
Pressure as a Measure of Concentration
For gases, pressure is not an abstract force; it is the result of gas molecules colliding with the walls of their container.
The Ideal Gas Law shows a direct relationship between pressure (P) and the number of moles (n) in a given volume (V) at a constant temperature (T). More molecules in the same space mean more pressure. Therefore, increasing pressure is functionally the same as increasing concentration.
The Role of Collision Theory
Chemical reactions occur when reactant particles collide with two key conditions met:
- They must have enough kinetic energy to overcome the activation energy barrier.
- They must collide with the correct physical orientation.
A collision that meets both criteria is called an effective collision. The rate of a reaction is determined by the frequency of these effective collisions.
More Pressure = More Collisions
When you increase the pressure of a gaseous system, you are forcing the same number of molecules into a smaller volume.
This higher density means the average distance between molecules decreases. Consequently, the total number of collisions between reactant molecules per unit of time increases significantly.
The Impact on "Effective" Collisions
While increasing pressure increases the total number of collisions, it does not change the kinetic energy of the individual molecules (assuming temperature is constant).
Therefore, the percentage of collisions that are effective remains the same. However, because the total number of collisions has gone up, the absolute number of effective collisions per second also increases. This is what drives the reaction rate higher.
When Pressure Does Not Affect the Rate
The link between pressure and reaction rate is powerful, but it is not universal. It's critical to know when it doesn't apply.
Reactions in Liquid and Solid Phases
Liquids and solids are considered incompressible phases. Applying external pressure does not significantly change their volume or the spacing between their constituent particles.
Because pressure changes don't alter the concentration of reactants in liquids and solids, pressure has a negligible effect on the rates of reactions occurring solely in these phases.
Reactions with No Gaseous Reactants
If a reaction mechanism involves multiple steps, the overall rate is determined by the slowest step, known as the rate-determining step.
If this rate-determining step does not involve any gaseous reactants, changes in external pressure will not influence the overall reaction rate.
Understanding the Trade-offs and Nuances
Controlling pressure is a common industrial strategy, but it comes with important considerations that go beyond simple rate changes.
Distinguishing from Temperature Effects
In practice, compressing a gas increases its temperature. Both higher pressure and higher temperature increase reaction rates, but for different reasons.
It is crucial to isolate the variables. Pressure increases the frequency of collisions, while temperature increases the energy and force of those collisions, making a higher percentage of them effective.
Le Châtelier's Principle and Equilibrium
For reversible reactions that reach a state of equilibrium, pressure plays a dual role. According to Le Châtelier's Principle, increasing the pressure will shift the equilibrium position to favor the side of the reaction with fewer moles of gas.
This is a separate concept from kinetics (rate). Pressure increases the rate of both the forward and reverse reactions, but it may shift the final balance of products and reactants.
The Engineering Cost of High Pressure
Building and maintaining high-pressure reactors is technologically demanding and expensive. The materials must be strong enough to withstand the stress, and extensive safety systems are required to prevent catastrophic failures. This economic and safety trade-off is a major factor in industrial process design.
Applying This to Your Goal
Your approach to using pressure depends entirely on the chemical system you are working with and what you are trying to achieve.
- If your primary focus is maximizing reaction speed for a gaseous process: Increasing pressure is a direct and effective method, provided you can manage the associated temperature changes and engineering costs.
- If you are studying chemical equilibrium: Remember that pressure affects both the reaction rates and the final equilibrium position, favoring the side with fewer gas molecules.
- If your reaction involves only liquids or solids: Changing the external pressure is not a viable strategy for controlling the reaction rate; focus on temperature, concentration, or catalysts instead.
By understanding pressure's direct link to concentration, you gain a powerful lever for controlling the kinetics of gaseous reactions.
Summary Table:
| Effect of Pressure on Reaction Rate | Key Takeaway |
|---|---|
| Gaseous Reactions | Increases rate by increasing concentration and collision frequency. |
| Liquid/Solid Reactions | Negligible effect; phases are incompressible. |
| Reaction Equilibrium | Shifts equilibrium to favor the side with fewer gas moles. |
| Industrial Application | Effective but requires robust, high-pressure reactor systems. |
Need precise control over your reaction conditions? KINTEK specializes in high-pressure lab reactors and equipment designed for reliable and safe experimentation. Whether you're optimizing a gaseous reaction rate or studying chemical equilibrium, our solutions deliver the performance and safety you need. Contact our experts today to find the perfect system for your laboratory's unique challenges.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Laboratory High Pressure Vacuum Tube Furnace
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS



















