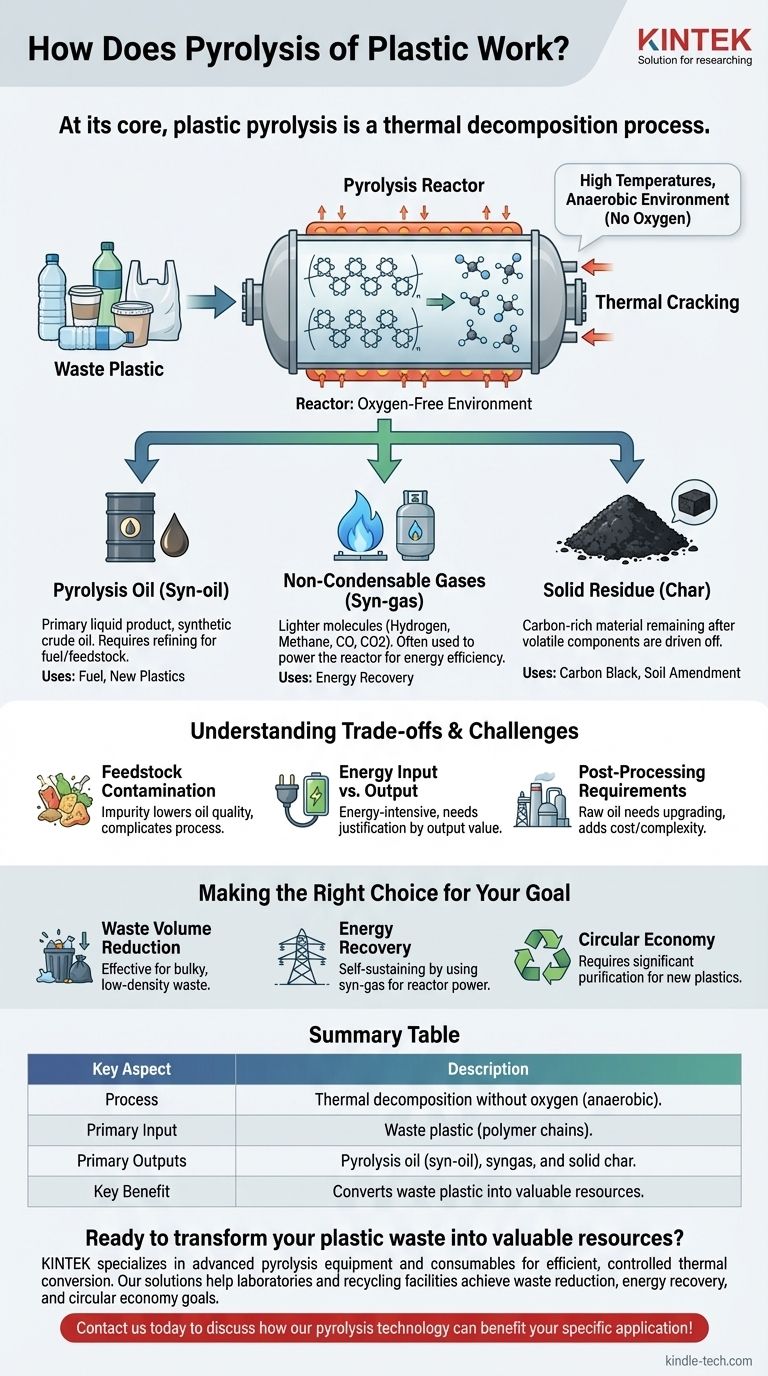
At its core, plastic pyrolysis is a thermal decomposition process. It uses high temperatures in an oxygen-free environment to break down the long, complex polymer chains that make up plastic. This controlled reaction effectively deconstructs the waste at a molecular level, converting it into smaller, more valuable components like synthetic oil, gas, and a solid residue.
Plastic pyrolysis isn't burning; it's a controlled chemical reaction that reverses the plastic creation process. The key is applying intense heat without the presence of oxygen, which forces the large polymer molecules to crack apart into simpler, useful substances rather than combust into ash and smoke.

The Core Mechanism: Deconstruction Without Oxygen
The Role of the Reactor
The entire process takes place inside a sealed, oxygen-deprived vessel called a pyrolysis reactor. Waste plastic is fed into this chamber, which is designed to withstand high temperatures and pressures.
The Critical Factor: Anaerobic Heating
The absence of oxygen (an anaerobic environment) is the most crucial element of pyrolysis. Without oxygen, combustion cannot occur. Instead of burning, the intense heat energy is absorbed directly by the plastic's molecular bonds.
The Chemical Reaction: Thermal Cracking
This absorption of energy causes the long polymer chains to vibrate violently and eventually break apart, or "crack." This is a process known as thermal cracking, similar to that used in petroleum refining but often at lower temperatures. The large, complex hydrocarbon molecules of the plastic are broken down into a variety of smaller, less complex molecules.
The Three Primary Outputs of Pyrolysis
Pyrolysis Oil (Syn-oil)
This is the primary liquid product and is often the main target output. It is a complex mixture of different hydrocarbons and can be thought of as a type of synthetic crude oil. This oil requires further refining before it can be used as a fuel or as a feedstock to create new chemicals and plastics.
Non-Condensable Gases (Syn-gas)
These are lighter molecules that do not condense into a liquid when cooled. This gas mixture typically includes hydrogen, methane, carbon monoxide, and carbon dioxide. In many modern systems, this syn-gas is captured and used to provide the energy needed to heat the reactor, making the process more energy-efficient.
Solid Residue (Char)
After the volatile components have been driven off as gas and liquid, a solid, carbon-rich material remains. This is known as pyrolysis char or carbon black. Its quality and potential uses depend heavily on the type and purity of the initial plastic waste.
Understanding the Trade-offs and Challenges
Feedstock Contamination
Real-world plastic waste is rarely pure. Contaminants like food residue, paper, and other types of plastic (like PVC, which releases corrosive hydrochloric acid) can significantly impact the process. These impurities can lower the quality of the pyrolysis oil and complicate the operation.
Energy Input vs. Output
Pyrolysis is an energy-intensive process that requires a significant thermal input to break the strong chemical bonds in plastics. For the process to be economically and environmentally viable, the energy value of the outputs must justify the energy required to run the system.
Post-Processing Requirements
The raw pyrolysis oil is not a "drop-in" fuel or feedstock. It often contains impurities and has properties that require it to be upgraded and refined before it can be used by traditional petrochemical infrastructure. This adds complexity and cost to the overall value chain.
Making the Right Choice for Your Goal
The specific parameters of a pyrolysis system are tuned based on the desired outcome.
- If your primary focus is waste volume reduction: Pyrolysis is highly effective, converting bulky, low-density plastic waste into a much denser liquid fuel and a small amount of solid char.
- If your primary focus is energy recovery: The process can become self-sustaining by using the produced syn-gas to power the reactor, with the syn-oil serving as a storable liquid fuel.
- If your primary focus is a circular economy: The pyrolysis oil must be viewed as a raw feedstock that requires significant purification to be suitable for creating new, high-quality plastics.
Ultimately, pyrolysis represents a powerful chemical engineering tool for redefining plastic waste as a resource rather than a liability.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Thermal decomposition without oxygen (anaerobic). |
| Primary Input | Waste plastic (polymer chains). |
| Primary Outputs | Pyrolysis oil (syn-oil), syngas, and solid char. |
| Key Benefit | Converts waste plastic into valuable resources. |
Ready to transform your plastic waste into valuable resources?
KINTEK specializes in advanced pyrolysis equipment and consumables for efficient, controlled thermal conversion. Our solutions help laboratories and recycling facilities achieve waste reduction, energy recovery, and circular economy goals.
Contact us today to discuss how our pyrolysis technology can benefit your specific application!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Mesh belt controlled atmosphere furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- Why must copper foil electrodes undergo high-temperature drying in a vacuum oven? Optimize Li6PS5Cl Cell Assembly
- What is the purpose of using high-temperature vacuum furnaces for annealing titanium dioxide? Optimize Bioactivity
- What are the advantages of using high-temperature industrial furnaces for thermal regeneration of spent carbon?
- What is the significance of vacuum heating for Li-IL in MOFs? Ensure Deep Dehydration & Battery Stability
- What is the main advantage of vacuum evaporation over atmospheric evaporation? Achieve Low-Temperature, High-Purity Processing
- Can brazing be used for ferrous metals? Yes, and here's how to ensure a strong joint.
- What is the structure of the electric arc furnace? A Detailed Breakdown of Its Core Components and Design
- Is physical vapor deposition top down or bottom up? A Guide to Bottom-Up Nanoscale Manufacturing



















