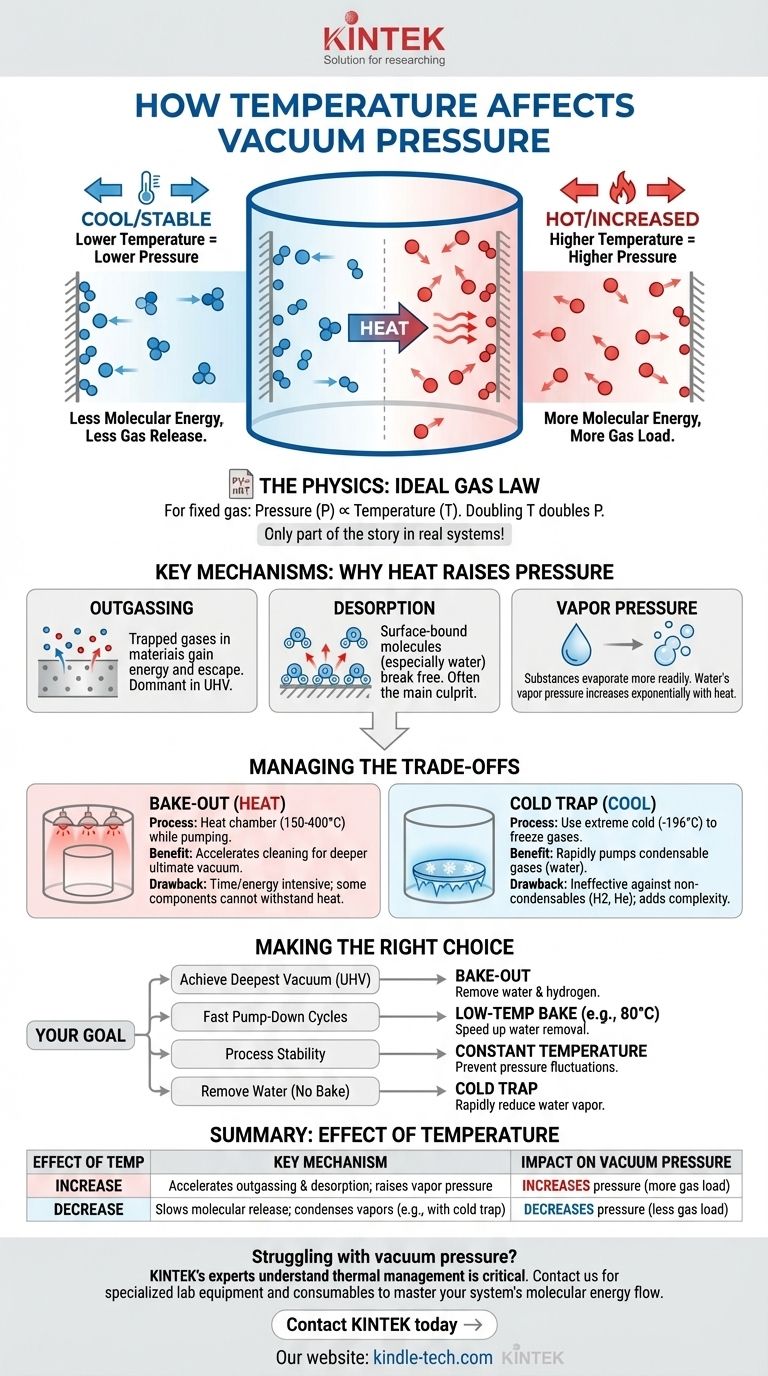
In a vacuum system, increasing the temperature almost always increases the pressure. This happens because heat gives energy to residual gas molecules, causing them to escape from the chamber surfaces and contaminants like water to evaporate more readily. This release of gas, known as the gas load, raises the system's pressure, making it more difficult for the vacuum pump to achieve or maintain a deep vacuum.
The core principle is this: temperature doesn't directly affect the vacuum itself, but it dramatically influences the behavior of molecules on the surfaces within your vacuum system. Higher temperatures increase the rate at which these molecules turn into gas, effectively working against your vacuum pump.

The Physics of Temperature and Pressure
To understand how to control your vacuum, you must first understand the relationship between heat energy and molecular behavior.
The Ideal Gas Law: The Foundation
The Ideal Gas Law (PV=nRT) provides the basic relationship. For a sealed container with a fixed amount of gas, pressure (P) is directly proportional to temperature (T).
Doubling the absolute temperature of the gas doubles the pressure. While this is a foundational concept, it only describes the behavior of gas already in the system, not the gas being added to it by other effects.
A Perfect vs. a Real Vacuum
In a theoretical, perfect vacuum with zero particles, temperature would have no meaning and no effect.
Real-world vacuum systems, however, are never perfectly empty. The pressure you measure is the result of residual gas molecules still moving within the chamber. Temperature's primary role is to determine how many of these molecules are released from the chamber walls and contaminants.
Key Mechanisms in Real-World Systems
In any practical vacuum system, the pressure is dominated by gas molecules that are not being pumped out fast enough. Temperature directly increases this "gas load" through three primary mechanisms.
Outgassing: The Hidden Gas Source
All materials, especially metals like stainless steel and aluminum, absorb gases from the atmosphere, primarily into the bulk of the material. This trapped gas is a hidden reservoir.
When you heat the chamber walls, you give these trapped molecules more kinetic energy. This energy allows them to migrate to the surface and escape into the vacuum, a process called outgassing. This is often the dominant gas load in high and ultra-high vacuum systems.
Desorption: Molecules on the Surface
Separate from outgassing, desorption refers to molecules (especially water) that are stuck to the surface of the chamber, not absorbed within it.
These molecules are held by weak physical bonds. A small increase in temperature can provide enough energy to break these bonds, releasing the molecules as gas and raising the pressure. Water is the most common culprit and is notoriously difficult to remove without heat.
Vapor Pressure: The Water Problem
Every liquid and solid has a vapor pressure, which is the pressure exerted when the substance is in equilibrium with its own vapor. This vapor pressure is extremely sensitive to temperature.
Water is the most significant contaminant in most vacuum systems. At room temperature, a single drop of water can prevent a system from reaching high vacuum. As you heat the system, water's vapor pressure increases exponentially, releasing a massive amount of gas that can overwhelm the pump.
Understanding the Trade-offs
Managing temperature is a balancing act. The two primary techniques, baking and cooling, have distinct benefits and drawbacks.
The "Bake-out"
A common procedure for high-vacuum systems is to bake out the chamber, often to temperatures of 150-400°C, while pumping.
- Benefit: Baking dramatically accelerates outgassing and desorption, driving off water and other contaminants much faster than at room temperature. After cooling down, the surfaces are significantly cleaner, resulting in a much lower ultimate pressure.
- Drawback: It is a time and energy-intensive process. Furthermore, many components like elastomer seals (O-rings), electronics, or optics cannot withstand high temperatures, limiting the applicability of a full system bake-out.
The "Cold Trap"
Conversely, you can use extreme cold to lower pressure. A cold trap or cryosurface is a surface within the vacuum system chilled to cryogenic temperatures, typically with liquid nitrogen (-196°C).
- Benefit: As gas molecules (especially water vapor) hit the cold surface, they freeze instantly, removing them from the system. This acts as a high-speed pump for condensable gases and can rapidly lower the pressure.
- Drawback: Cold traps are ineffective against non-condensable gases like hydrogen, helium, and neon. They also add complexity and operating cost (e.g., the need for liquid nitrogen).
Making the Right Choice for Your Goal
Controlling temperature is essential for achieving predictable and deep vacuum levels. Your strategy should align with your specific objective.
- If your primary focus is achieving the deepest possible vacuum (UHV): You must perform a bake-out to remove water and hydrogen from the chamber walls.
- If your primary focus is fast pump-down cycles for a high-vacuum process: A low-temperature bake (e.g., 80°C) combined with clean, low-outgassing materials will significantly speed up water removal.
- If your primary focus is process stability: You must ensure the chamber and all internal components are at a constant, controlled temperature to prevent pressure fluctuations from ruining your work.
- If you are struggling with water vapor in a system that cannot be baked: A cold trap is your most effective tool for rapidly reducing the partial pressure of water.
Ultimately, mastering your vacuum system means mastering the flow of molecular energy.
Summary Table:
| Effect of Temperature | Key Mechanism | Impact on Vacuum Pressure |
|---|---|---|
| Increase | Accelerates outgassing & desorption; raises vapor pressure | Increases pressure (more gas load) |
| Decrease | Slows molecular release; condenses vapors (e.g., with a cold trap) | Decreases pressure (less gas load) |
Struggling with vacuum pressure instability or slow pump-down times? The experts at KINTEK understand that precise thermal management is critical for your laboratory's success. Whether you need to achieve ultra-high vacuum with a controlled bake-out or rapidly remove water vapor with a cold trap, our specialized lab equipment and consumables are designed for reliability and performance.
Contact KINTEK today to discuss your specific vacuum challenges. Let us help you select the right equipment to master your system's molecular energy flow and achieve consistent, repeatable results.
Visual Guide
Related Products
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Laboratory High Pressure Vacuum Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What role does a high-temperature vacuum furnace play in the tungsten coating workflow? Optimize Stress Relief Today
- Why hydrogen is used in sintering furnace? The Key to Superior Purity and Strength
- What is the function of a resistance furnace in the preparation of ultrafine metallic uranium powder? Guide to HDH.
- What is vacuum deposition of metal? Achieve Atomic-Level Coating Control for Superior Performance
- What role does a high-temperature laboratory furnace play in studying the atomic ordering of Fe-Al powders? Achieve LRO
- What is the process of vacuum heat treatment? Achieve Superior Material Performance and Purity
- What is pyrolysis gasification in waste management? Transform Waste into Valuable Resources
- What is the function of a vacuum drying oven in the preparation of succinimide anion-based ionic liquids?



















